Blog
Unlocking New Targets: Cytokine Receptor Platform for Immunology Innovation
Cytokines and their receptors orchestrate intricate immune responses, yet when dysregulated, they fuel persistent inflammation and tissue damage, causing diseases like psoriasis and rheumatoid arthritis. Despite their potential, the pleiotropic nature of cytokines has historically led to dose-limiting toxicity or reduced efficacy in therapies, underscoring the need for precise engineering and pharmacological insights to unlock their full potential [1]. Today, comprehensive identification of cytokine-receptor interactions remains key to unlocking safer, more effective medicines.
We know your real struggles: navigating the redundancy and pleiotropy of cytokine signaling pathways that often result in off-target effects and unpredictable clinical outcomes. In addition, poor bioavailability, limited tissue penetration, and challenges in modulating downstream intracellular cascade can derail even the most promising candidates [2][3]. These hurdles demand not just innovation, but a rigorous, collaborative approach that cuts complexity with clarity and makes responsiveness your edge.
Recent advances in understanding immune-mediated inflammatory diseases – often without known etiology - have been propelled by targeted treatments, with cytokines and their receptors emerging as prime candidates for drug development [4]. Immunomodulators and novel targeted therapies have transformed disease management by specifically modulating immune pathways rather than broadly suppressing immune function. Immunomodulation can improve disease control and reduce side effects, as many aspects of cytokine function are highly tunable, but require an overarching understanding of its intricate molecular cascades [5].
ChemPartner’s Cytokine Receptor Platform: Integrated Solution for Drug Discovery
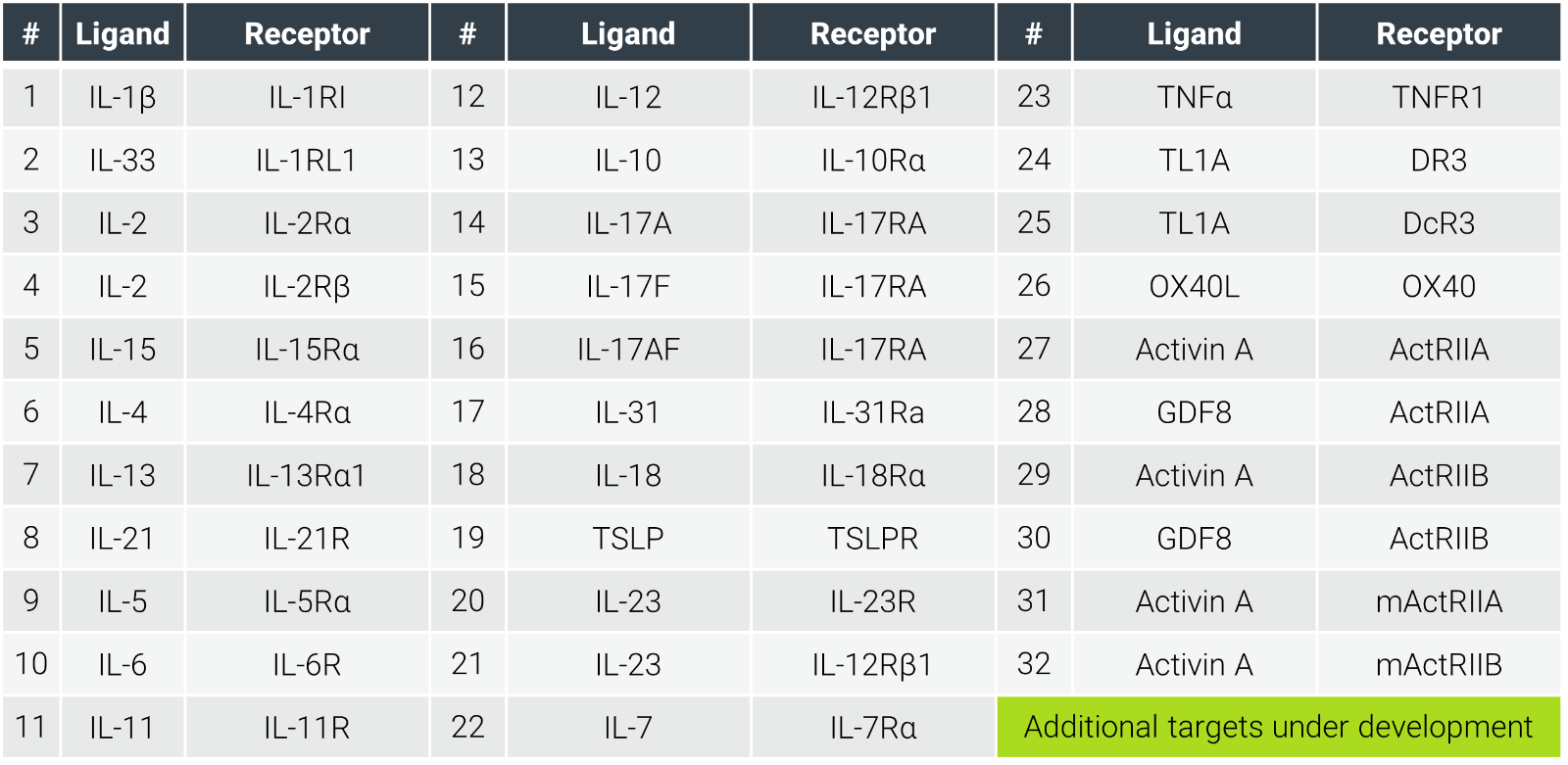
Traditional screening often limits options to a handful of assays, which leads to incomplete picture of the disease etiology. At ChemPartner, we address this with over 60 validated assays across cytokine families, enabling rigorous mechanistic evaluation and seamless progression to preclinical studies.
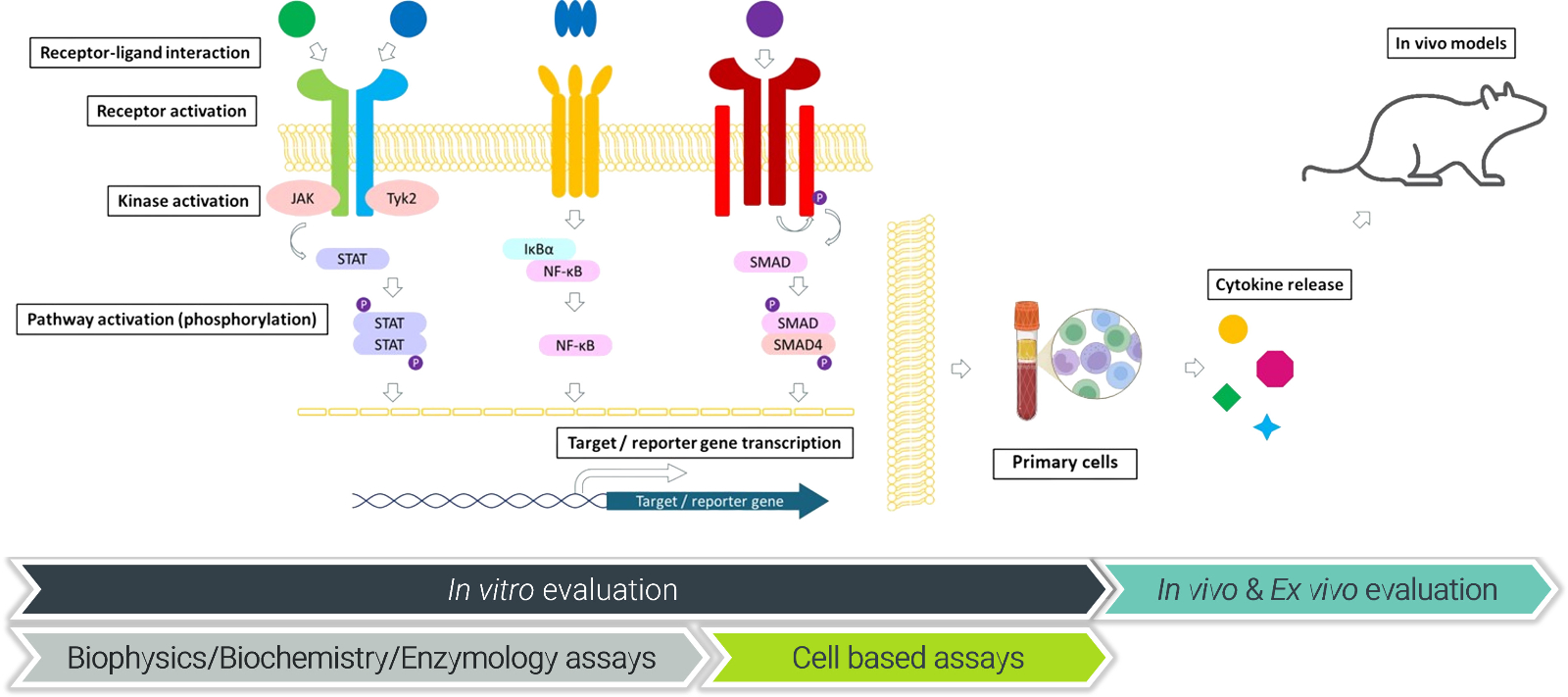
We’ve built this platform to span cytokine receptor biology comprehensively, from biophysical binding to the in vivo validation and PK/PD studies, ensuring actionable data that can advance your candidates efficiently. We are targeting the key elements of the therapeutic puzzle: for example in psoriasis, intricate interactions between immune cells and cytokines - including interleukins (ILs), interferons (IFNs), tumor necrosis factors (TNFs), chemokines, and transforming growth factor-β (TGF-β), which all collectively define the chronic inflammatory condition, controlling cellular activity and immunological responses while contributing to pathogenesis [6].
SCHEDULE A CALL WITH A SCIENTIST
Our platform is constantly expanding, including emerging targets like TNF-like cytokine 1A (TL1A) and its functional receptor, death-domain receptor 3 (DR3), which play key roles in regulating innate and adaptive immune responses, being now recognized as a new frontier of IBD targeted therapies. [7]
Biophysical and Biochemical Assays for Foundational Insights
Our platform offers deep insights into receptor-ligand interaction and measurement of binding affinity using SPR, TR-FRET, and cellular binding assays. Each assay is extensively validated with reference compounds such as dupilumab and mirikizumab, which confirm yield nanomolar IC50s in IL-4 assays, demonstrating high selectivity.
Ligand-Induced Receptor Activation (LIRA): TR-FRET ternary assays assess co-receptor recruitment, with examples like mirikizumab in IL-23 and tezepelumab in TSLP, providing clear activation profiles.
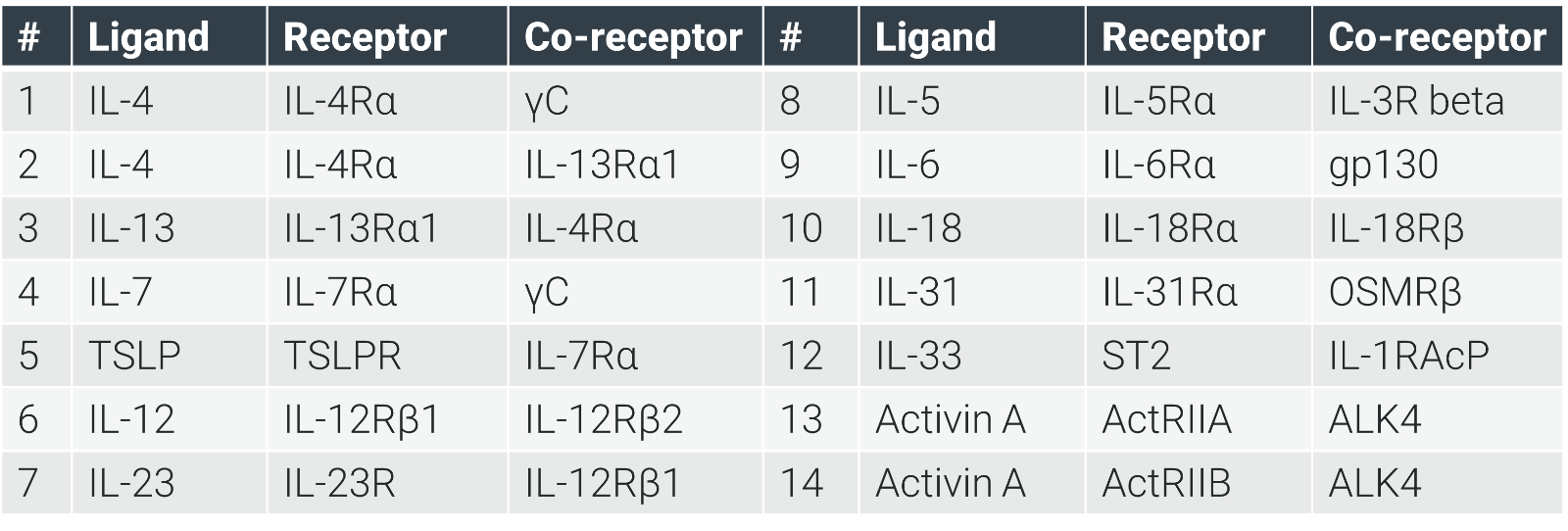
Intracellular Downstream Signaling: Kinase assays target JAK/TYK, while STAT-DNA binding and phosphorylation in cell lines (e.g., IL-4-pSTAT6) deliver precise readouts, supported by luminescence, MSD, and FACS.
Primary Cell and Functional Assays for Translational Relevance
In primary cells like PBMCs, whole blood, or splenocytes across species (human to mouse), we can evaluate pSTAT responses to IFNα, IL-6, or IL-23, for example. Cytokine release via MSD or CBA measures endpoints like IL-4/IL-13-induced CCL17 or TL1A-induced IFNγ, with IC50 data for inhibitors like tofacitinib and baricitinib.
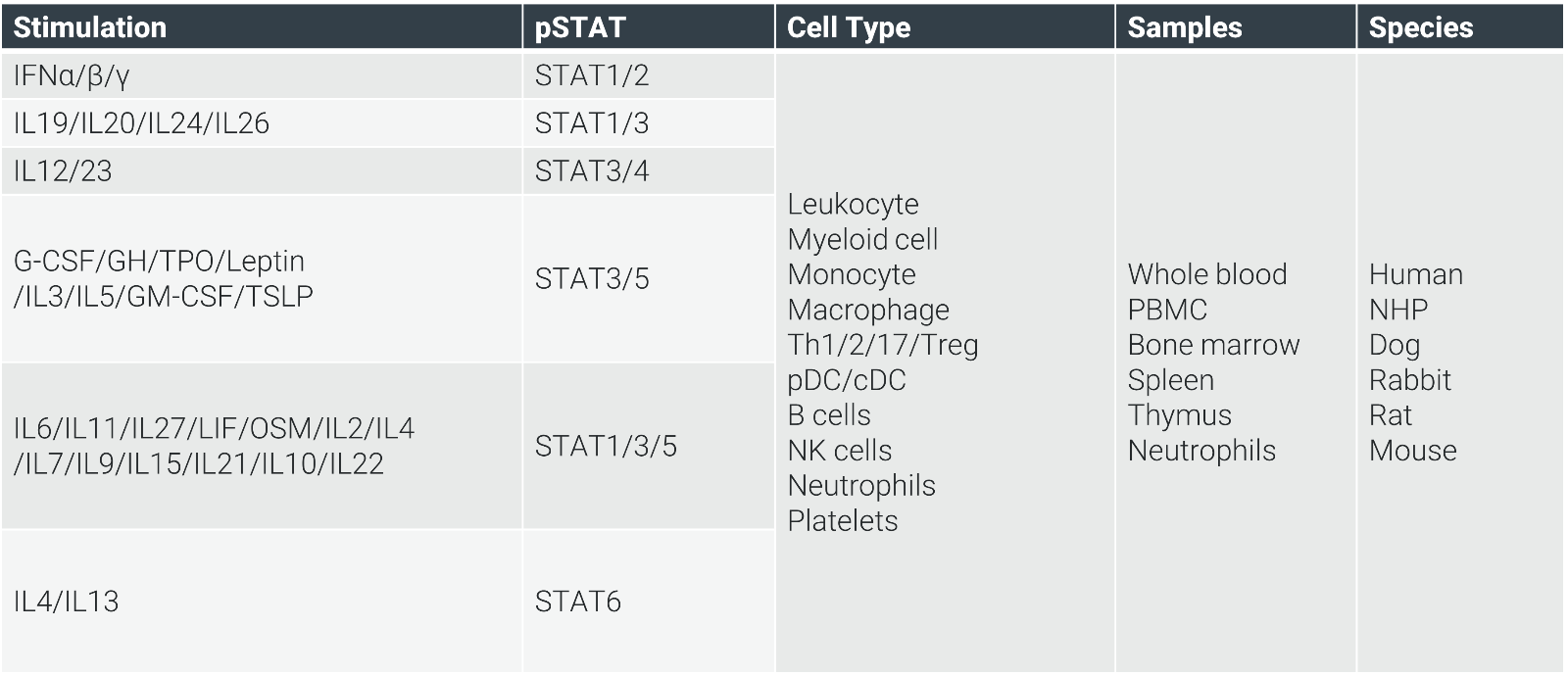
In Vivo Models for Bridging Discovery to Clinic
With over 30 validated in vivo models tailored to mimic cytokine-driven diseases, ChemPartner's platform bridges discovery to clinic through rigorous, versatile evaluation in relevant physiological contexts. From respiratory conditions, RA, MS, IBD, skin disorders such as psoriasis and dermatitis models, delayed-type hypersensitivity, and systemic inflammation, these models integrate pharmacodynamic and pharmacokinetic endpoints to assess exposure, engagement, and modulation efficiently.
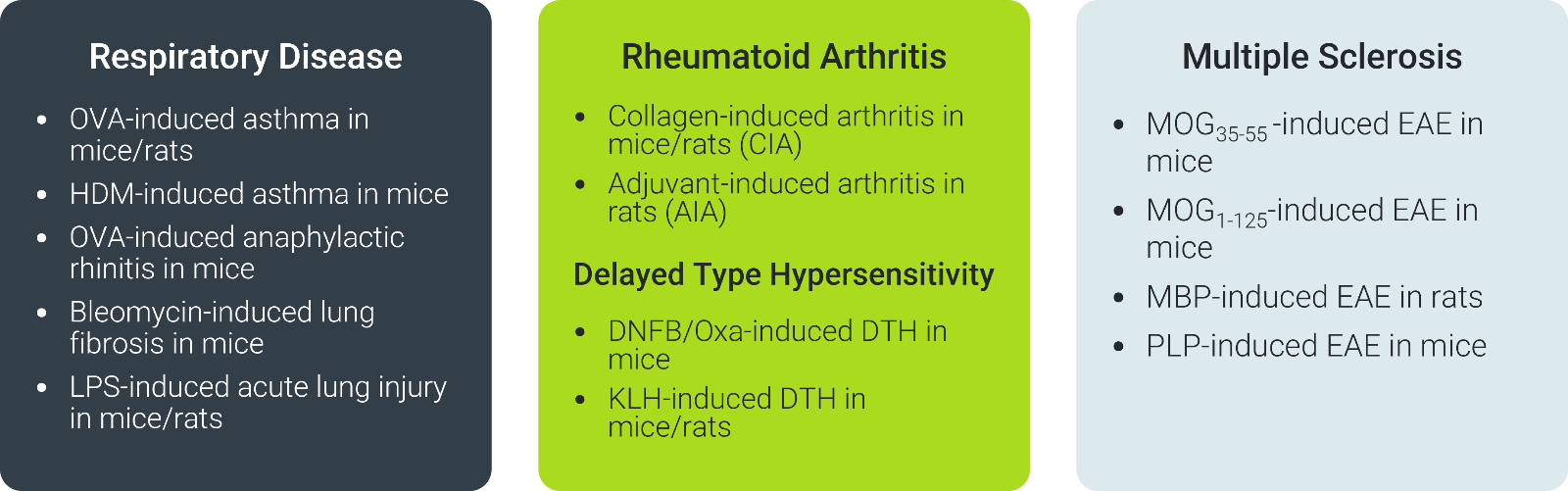
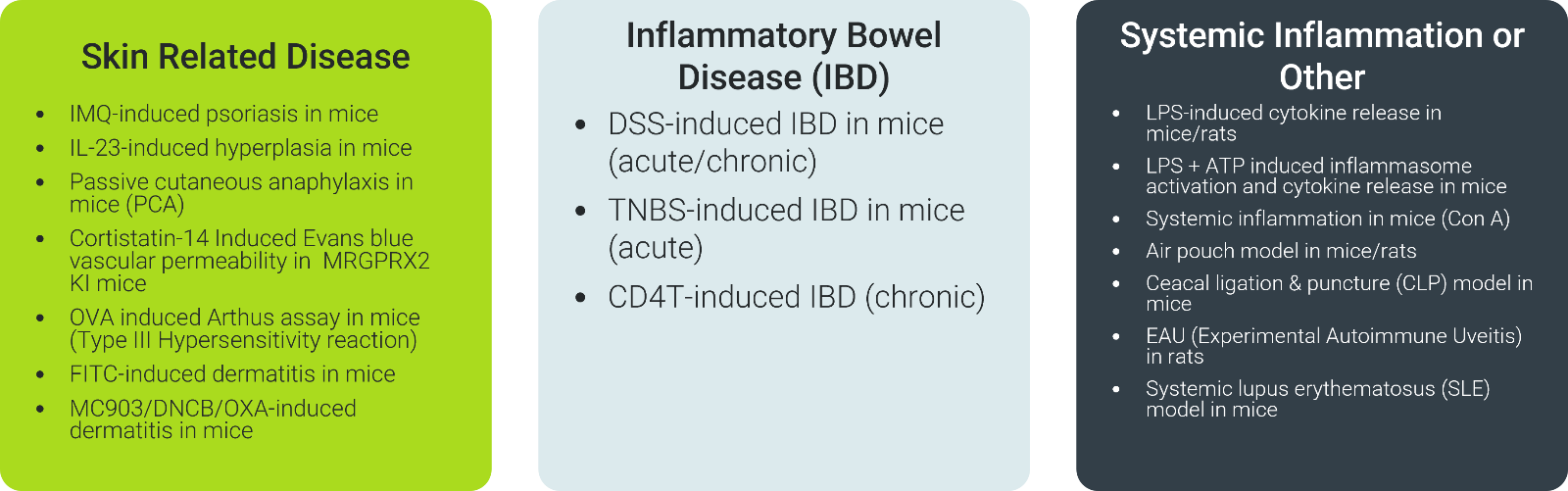
ChemPartner: Catalytic Innovation on Your Terms
In a field where every second counts and evidence is everything, ChemPartner catalyzes personal connections through our 2,000 detail-obsessed scientists, delivering versatile, rigorous support that flexes to fit your partnership. Whether you need strategic guidance to navigate cytokine redundancy or rapid execution to optimize pharmacokinetics and de-risk your candidates, we are listening.
Are you targeting IL-17 for psoriasis or JAK for RA? Our collaborative team is eager to push your science forward, cutting complexity with clarity to accelerate breakthroughs on your terms!
DISCUSS YOUR PROJECT WITH A SCIENTIST
Bibliography
[1] Saxton RA, Glassman CR, Garcia KC. Emerging principles of cytokine pharmacology and therapeutics. Nat Rev Drug Discov. 2023;22(1):21-37. doi:10.1038/s41573-022-00557-6
[2] De Vita V, Scolamiero G, Santinelli C, et al. From Large to Small Cytokine Receptor Antagonists. SLAS Discov. 2025;30(7):100162. Published 2025 Oct 4. doi:10.1016/j.slasd.2025.100162
[3] Zhang Y, Lin S, Cotterell J, et al. A kinase to cytokine explorer to identify molecular regulators and potential therapeutic opportunities. eLife. 2024;13:e91472. Published 2024 Feb 2. doi:10.7554/eLife.91472
[4] Takeuchi T. Cytokines and cytokine receptors as targets of immune-mediated inflammatory diseases-RA as a role model. Inflamm Regen. 2022;42(1):35. Published 2022 Dec 1. doi:10.1186/s41232-022-00221-x
[5] Yadav, L. Sahana1; Samruddhi, H. S.1; Raju, Akondi Butchi2; Mallamma, T.1. Cytokines, Kinases, and Beyond: Advancing Next-generation Immunomodulatory Strategies and Precision Small-molecule Therapies in Autoimmune Disease. Asian Journal of Pharmaceutical Research and Health Care 17(3):p 209-219, Jul–Sep 2025. | DOI: 10.4103/ajprhc.ajprhc_201_25
[6] Li L, Liu J, Lu J, et al. Interventions in cytokine signaling: novel horizons for psoriasis treatment. Front Immunol. 2025;16:1573905. Published 2025 Apr 15. doi:10.3389/fimmu.2025.1573905
[7] Bamias G, Menghini P, Pizarro TT, Cominelli F. Targeting TL1A and DR3: the new frontier of anti-cytokine therapy in IBD. Gut. 2025;74(4):652-668. Published 2025 Mar 6. doi:10.1136/gutjnl-2024-332504