Blog
More Than the Sum of Parts: Dual-Payload ADCs and the Art of DAR Optimization
Antibody Drug Conjugates (ADCs) combine targeting precision of monoclonal antibodies with cytotoxic agents. They selectively deliver a highly toxic payload to tumors, which prevents systemic toxicity, offering higher therapeutic index and reduced risk of side effects. Recent decades brought breakthroughs in antibody engineering and sophisticated linker and payload chemistry, resulting in ADCs becoming one of the fastest developing drug classes in oncology. Biopharma companies are actively developing novel modalities such as bispecific, biparatopic or even multispecific ADCs that can bind to two distinct cancer cell receptors and even recruit immunomodulating cells at the same time. All that while delivering a deadly payload to the tumor. Read more on multispecific antibodies in our previous blog post One Antibody, Many Tasks: The Rise of Multi-Specific ADCs
Dual-payload ADCs deliver two different cancer drugs via one antibody, offering future potential to boost efficacy and overcome resistance.
Dual-Payload ADCs: Solution for Cancer Heterogeneity and Payload Resistance?
ADCs technology is not free from hurdles, such as linker stability and precise payload release. The complexity of cancer disease and nonhomogeneous nature of tumor microenvironment present additional obstacles. In our previous blog titled Engineering Smarter ADCs: Novel Linkers for Better Safety and Efficacy, we discussed how tandem cleavage linkers can be used to address premature payload release. Here, we would like to focus on heterogeneity and the acquired resistance of cancer cells.
Tumor cells can often exhibit variable antigen expression across cell populations, for example, antigen-positive cells can be surrounded by neighboring heterogeneous populations that lack target antigen expression whatsoever. In addition, tumor cells can develop drug resistance through upregulating efflux pumps, removing the drug, or by the target antigen downregulation, which prevents the ADCs from binding and internalizing the toxin in the first place. Cancer cells are also well known to evade apoptosis cascades.
This has led to the emergence of dual-payload ADCs (dpADCs), intricately tuned biologics with two toxins working in either synergistic or additive mode. For example, a single mAb can be conjugated with a topoisomerase I inhibitor, such as deruxtecan, and in addition sport a microtubule inhibitor, like Monomethyl Auristatin E (MMAE). Constructed this way, the ADC can attack cancer cells through orthogonal pathways, helping to overcome resistance. DpADCs can also take advantage of the bystander killing effect, where a cell permeable payload and a cell-resident payload are conjugated to the same ADC to address uneven antigen expression. An example of this approach might use DXd, a cell permeable topoisomerase I inhibitor (utilized in the approved Enhertu), or EG5 pyridine inhibitor, a potent antimitotic agent. [1][2]
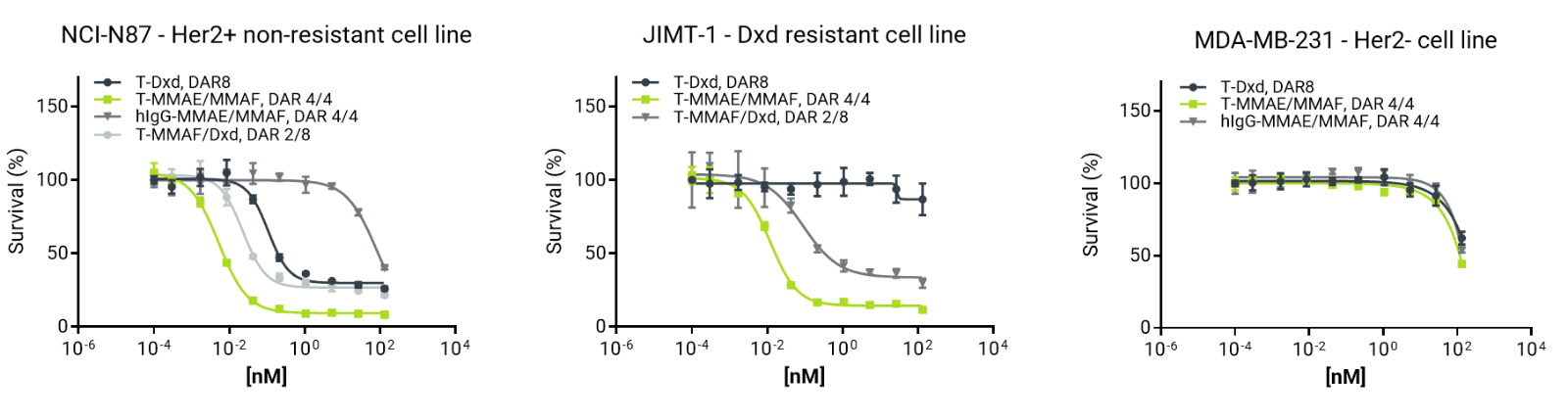
Cytotoxic activity of trastuzumab ADCs across HER2+ and HER2– cell lines. In HER2+ non‑resistant cells, all trastuzumab ADCs induced cell death. In contrast, in DXd‑resistant HER2+ cells, cytotoxicity was observed only with the dual‑payload ADC. No activity was detected in HER2– control cell lines, consistent with target‑dependent effects.
Dual-payload ADCs and DAR: Simple Math Doesn’t Apply
Two single-payload ADCs will work differently than a mAb equipped with two payloads. Preclinical studies involving tumor models with HER2 and TROP2 as target antigens showed that a dpADC equipped with both MMAE and MMAF outperformed either single-payload ADC alone or co-administered two single-payload ADCs targeting the same antigen.[3] Obviously, these results can be attributed to the fact that the two separate single payload ADCs compete for the same antigen, which may result in non-synchronized and non-homogeneous internalization. Higher affinity ADC would prevail in delivering one type of payload over the inferior affinity antibody. However, the situation is more nuanced, and drug-antibody ratio (DAR) plays a crucial role in the performance of both single and dual-payload ADCs.
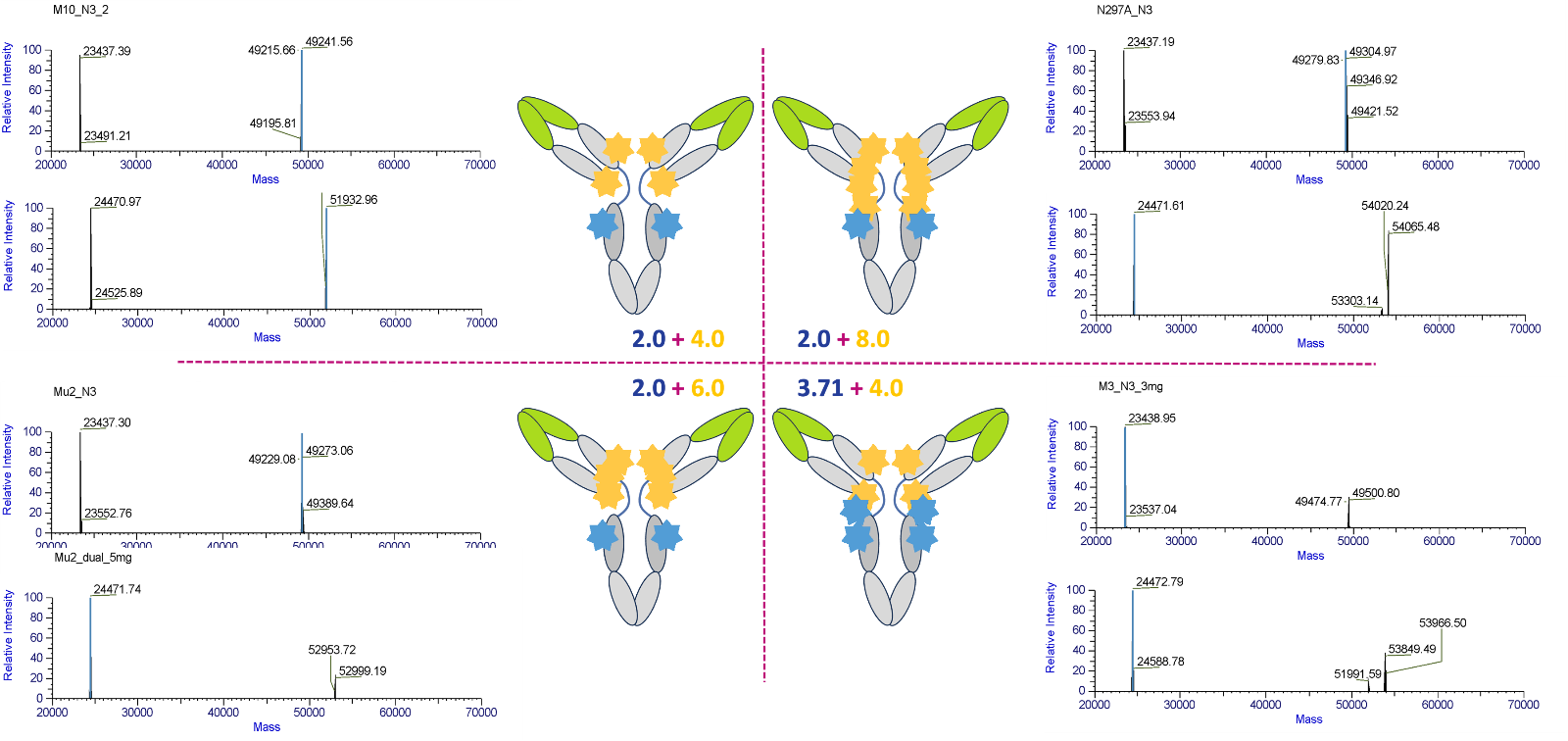
Demonstration of controlled ADC engineering. Clean mass spectrometry profiles confirm the generation of single, well-defined conjugate species with predictable DARs.
Both DAR and the dual payload ratio can critically impact clinical performance of ADCs, altering their efficacy, toxicity, and pharmacokinetic properties. Just like dual-payload ADCs have the potential to offer combined mechanisms of action (MOAs) therapeutic effect, they can also cause additive or synergistic toxicities. So, in addition to achieving a uniform DAR of 4 or 8, dual-payload systems must control both total DAR and the relative ratio between both payloads. Thus, a DAR of 4+2 can in addition offer payload distribution of 4+2, 3+3, 5+1 and other combinations impacting efficacy and safety. As an example, during a study investigating anti-TROP2 dpADC with a topoisomerase I inhibitor and RNA polymerase II inhibitor a very specific DAR ranges were established (5.8-6.2) with tight payload ratio tolerances (4±0.2:2±0.2). This highlights the need to control the conjugation chemistry closely. [1] These specifications ensure batch-to-batch consistency since the arbitrary increase of total DAR of dual payload conjugates requires fine tuning to regulate hydrophilicity, sustain therapeutic plasma half-life, mitigate aggregation, and maintain formulation stability. Ultimately, fine tuning DAR and the payload ratio are critical for achieving optimal synergy between the two distinctive MOAs delivered by each toxin.
From Concept to Clinic: Balancing Biological Ambition with Practical Constraints
As we discussed dual-payload ADC technology is not without challenges, and it is not simply “two single-payload ADCs on one molecule”. Maximizing total DAR without payload ratio optimization can surely compromise pharmacokinetics and solubility and great care must be taken to avoid mismatched payload release kinetics. Despite these concerns, dpADCs offer several significant advantages over single payload therapeutic mABs. Dual-payload ADCs can synergistically exploit two MOAs resulting in better therapeutic efficacy by simultaneously releasing both payloads. All that without target-antigen competition if two different ADCs were co-administered. In theory, dpADCs have promising potential to combine with other therapeutic agents, namely clinical potential for combination with therapies such as immune checkpoint inhibitors, as well as in conjunction with chemotherapy, radiotherapy, cell therapy and other targeted therapies. Finally, developing a dual-payload ADC could be less complex than pursuing two single-payload ADCs in parallel, simplifying regulatory pathways, clinical development, and manufacturing through one data package, dose-escalation study, and CMC campaign. In addition, a dual-payload ADC streamlines treatment logistics and regulatory requirements by eliminating the need to coordinate and optimize co-administration of multiple ADCs.[4][5]
From Complexity to Clarity: Advancing Dual-Payload ADC Design
At ChemPartner, with two decades of sponsor-grade discovery experience, we approach dual-payload ADC design deliberately, rigorously, and with clinical reality in mind. By expertly controlling DAR and payload ratios, we help our partners harness synergy without sacrificing developability or safety. Together, we navigate complexity with clarity, transforming ambitious ADC concepts into programs built to perform in the real world - and ultimately, to reach patients faster.
Bibliography
[1] Dixit R. Challenges and future directions of dual payload antibody-drug conjugates. Bioconjugation Insights. 2025;1(4):137-141. doi:10.18609/bci.2025.025
[2] Wang Z, Li H, Gou L, Li W, Wang Y. Antibody-drug conjugates: Recent advances in payloads. Acta Pharm Sin B. 2023;13(10):4025-4059. doi:10.1016/j.apsb.2023.06.015
[3] Yamazaki CM, Yamaguchi A, Anami Y, et al. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat Commun. 2021;12(1):3528. Published 2021 Jun 10. doi:10.1038/s41467-021-23793-7
[4] Wen M, Yu A, Park Y, Calarese D, Gerber HP, Yin G. Homogeneous antibody-drug conjugates with dual payloads: potential, methods and considerations. MAbs. 2025;17(1):2498162. doi:10.1080/19420862.2025.2498162
[5] Tsuchikama K, Anami Y, Ha SYY, Yamazaki CM. Exploring the next generation of antibody-drug conjugates. Nat Rev Clin Oncol. 2024;21(3):203-223. doi:10.1038/s41571-023-00850-2