Modern Drug Discovery Workflows
There are several reasons why it is important to introduce in vitro absorption, distribution, metabolism, and excretion (ADME) assays as early as possible into a modern drug discovery workflow, especially for biotech companies.
Here are three key reasons to introduce ADME studies as soon as possible:
1. Early Identification of Drug Candidates with Favorable ADME Profiles
ADME studies play a crucial role in identifying drug candidates with desirable pharmacokinetic properties. By assessing how a potential drug is absorbed, distributed, metabolized, and excreted in vitro, companies can obtain valuable information on its bioavailability, potential toxicity, and the likelihood of achieving therapeutic concentrations at the target site.
Identifying drug candidates with favorable ADME profiles early on allows companies to prioritize and allocate resources to the most promising compounds, thus increasing the chances of success in later stages of drug development.
2. Reduce Late-Stage Failures and Costly Setbacks
Introducing ADME assays early in the drug discovery workflow helps minimize late-stage failures and costly setbacks or delays. By identifying potential ADME-related issues at an early stage, biotech companies can eliminate or modify drug candidates that exhibit poor absorption, extensive metabolism, inadequate distribution, or slow excretion.
This early assessment helps avoid investing substantial resources in compounds with inherent ADME limitations that are unlikely to progress successfully through preclinical and clinical development. By reducing late-stage failures, biotech companies can save significant time, effort, and costs associated with failed drug candidates.
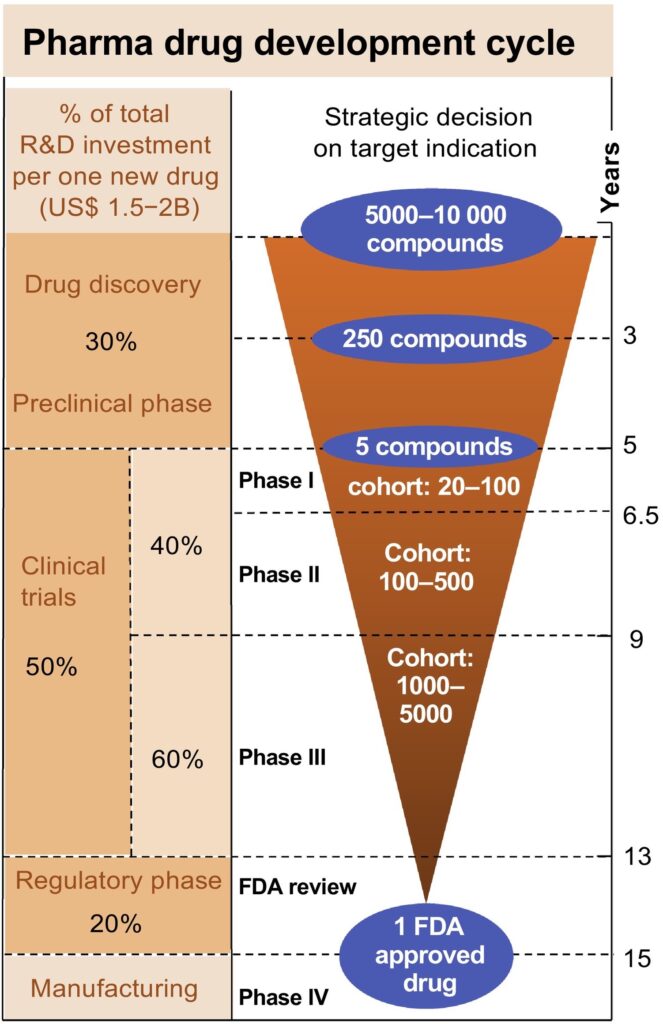
The graphic here, published in 2019, shows a 15 year timeline for the costs associated with developing a new drug by category. You can see that the discovery and pre-clinical stage makes up 30% of the overall drug development spend. Not an insignificant number if we’re talking about $2 billion overall to bring a new medication to market.
To put it bluntly, “killing” compounds quickly, often, and early in discovery is much more cost-effective than killing later in development after more substantial time and resources have been spent.
3. Compliance with Regulatory Requirements
Regulatory agencies such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) require thorough characterization of a drug candidate’s ADME properties during the drug development process. Introducing ADME assays early allows companies to gather essential data needed for regulatory submissions.
By providing comprehensive ADME information, including evidence of appropriate metabolism, favorable pharmacokinetics, and acceptable routes of elimination, companies can enhance the chances of obtaining regulatory approval for their drug candidates. This early compliance with regulatory requirements helps expedite the drug development process and brings potential therapies closer to market introduction.
Overall, incorporating in vitro ADME assays or conducting in vitro ADME studies early in the drug discovery workflow offers companies significant advantages that save time, money, and headaches.