Watch our on-demand webinar:
Biological Macromolecules: Exploring Analytical Development and QC Case Studies in Antibody Developability and Biologics CMC
Presented by Dr. Jinghan Su, Assistant Director and Head of Developability, and Dr. Qian Sun, Director, Head of Analytical Development and Quality Control at ChemPartner.
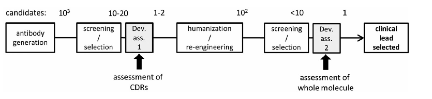
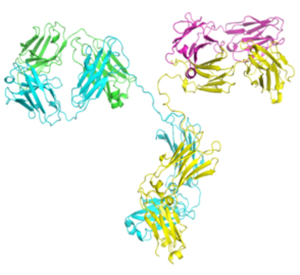
- • 潜在的CDR热点分析
- • 生物物理表征
- • 稳定性研究
- • 高浓度可能性评估