Three-decade long saga brings promise and breakthrough for better therapies
Kirsten rat sarcoma (KRAS) has been long recognized as the most commonly mutated protooncogene, and under active exploration in cancer research for the past 30 years. The target has been at some point deemed undruggable due to lack of typical binding pockets that would allow small molecule drugs to bind and inhibit its activation. This meant very limited options for patients with mutated KRAS who had not responded to chemotherapy or immunotherapy. Scientist have tried to target KRAS indirectly, for example by disrupting KRAS post-translational modifications by blocking farnesyltransferase, or via inhibition of SOS1, a key activator, promoting the exchange of GDP for GTP. Extensive research effort has been devoted to blocking KRAS downstream effectors such as RAF, MEK, ERK, or PI3K with dozens of inhibitors currently under clinical evaluation [1].
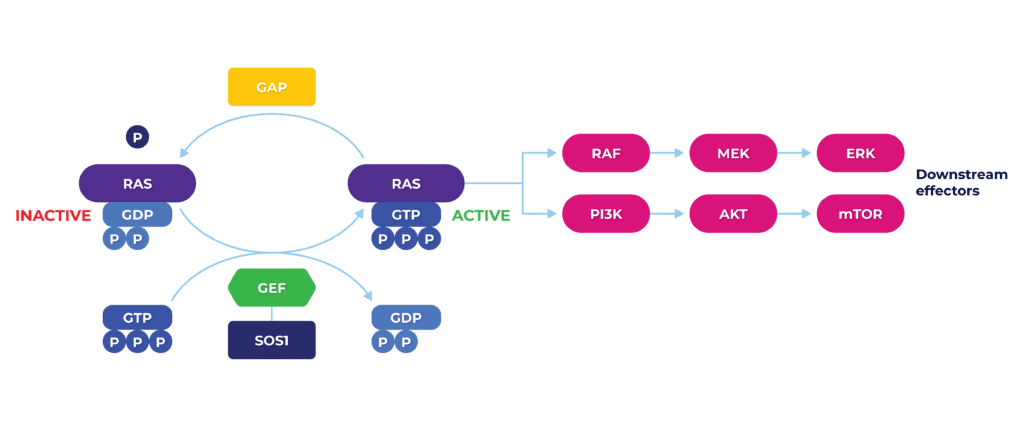
KRAS, in physiological equilibrium, acts as a signal transducer, ultimately regulating cell proliferation, growth, differentiation and apoptosis via multielement molecular cascade. Mutations such as KRASG12C tip the scale towards the persistence of the GTP-bound active state, overwhelming downstream signaling and leading to uncontrolled cell growth and oncogenesis.
In the recent years, renewed and successful efforts have been made to target the mutant KRAS directly. Scientist were able to achieve that by finding a unique, previously undiscovered, binding spot on the surface of the mutated protein, with promising efficacy results in clinical studies. In 2021 Amgen’s sotorasib (sold as Lumakras), first in class KRASG12C inhibitor has been fast-track approved to treat non-small cell lung cancer (NSCLC). A year later, adagrasib (sold as Krazati) from Mirati/BMS has also been approved for patients with the same, pretreated metastatic disease. Both drugs have comparable efficacy and potential in treating cancer that has spread to the brain [2].
Undruggable no more: a new era of combination therapies for patients
Being able to directly inhibit mutated KRAS has been hailed as a major breakthrough in oncology. However, despite promising results, cancer often develops resistance to KRAS inhibition alone. Cancers often re-establish oncogenic signaling through multiple mechanisms, including increased activation of mutant KRAS or strengthening of bypass events. However, druggability of mutated KRAS brings a whole new therapeutic universe of combination therapies to co-target both upstream co-regulators of KRAS and its downstream effectors. Combination approaches have been extensively investigated since availability of KRAS G12C direct inhibitors, including receptor tyrosine kinases (RTKs) and SHP2, with an improvement of response in preclinical models. Also, in KRASG12C-mutant NSCLC and CRC models, co-administering SOS1 inhibitor enhanced the anticancer response [3].
Dedicated to Life Science: ChemPartner provides reliable preclinical assay platforms for KRAS G12C targeted therapy, including / model for brain metastases
We have developed a comprehensive KRAS biochemical assay platform that includes GTP association assay, KRAS activity assay, KRAS-SOS1, KRAS-PI3K, and KRAS-RAF binding assays, facilitating fast and reproducible screening of new KRAS-targeted inhibitors. We tested our platform using AMG 510 (sotorasib) to verify the platform’s clinical relevance.
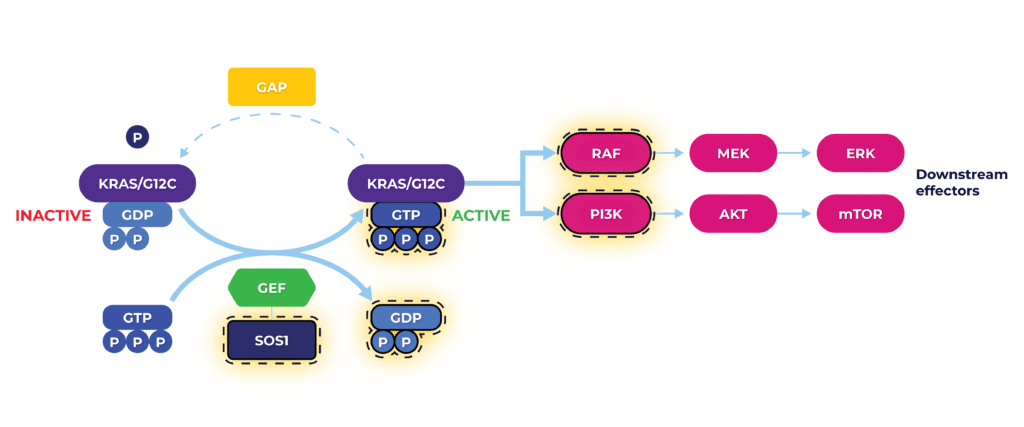
KRAS Assay Platform evaluates key elements of mutated KRAS molecular pathway (highlighted) for testing inhibitory effect of compounds targeting KRAS G12C. In addition, we have also developed 2D and 3D cell proliferation assays to determine the potency of AMG 510 in KRAS G12C mutant cell lines.
To evaluate the / activity of AMG 510 for NSCLC brain metastasis treatment and gain insights for developing new KRAS-targeted inhibitors, we have also established challenging orthotopic brain models. We inoculated mice with the NCI-H358-luc cell line – a NSCLC cell line which harbors the KRAS G12C mutation. We established intracranial, intracarotid and intracardiac metastatic models, and each model was validated with AMG 510 as a single agent and/or combined with radiation, a common approach in brain tumor therapy. Preliminary results from our / models suggest that AMG 510 can penetrate the blood-brain barrier and demonstrate therapeutic activity against KRAS G12C metastatic brain tumors. We consistently confirmed this using the intracarotid and intracardiac models that leave the blood-brain barrier intact. Furthermore, using the intracranial model, we demonstrated that the combination of AMG 510 with radiation increased efficacy compared to radiation alone.
Results were presented at 7th Swedish Cancer Research Meeting in Malmö, Sweden, 22-23 May, 2025
REQUEST THE POSTER BY CLICKING ON THE IMAGE BELOW:
References
[1] Ryan MB, Corcoran RB. Therapeutic strategies to target RAS-mutant cancers. Nat Rev Clin Oncol. 2018;15:709–20.
[2] Gadgeel SM, Jänne PA, Spira AI, et al. KRYSTAL-1: two-year follow-up of adagrasib (MRTX849) monotherapy in patients with advanced/metastatic KRASG12C-mutated NSCLC. Presented at: International Association for the Study of Lung Cancer 2023 World Conference on Lung Cancer; September 9-12, 2023; Singapore.
[3] Thatikonda, V., Lyu, H., Jurado, S. et al. Co-targeting SOS1 enhances the antitumor effects of KRASG12C inhibitors by addressing intrinsic and acquired resistance. Nat Cancer 5, 1352–1370 (2024).