ChemPartner brings awareness about Autoimmune & Autoinflammatory Arthritis on 2025 AiArthritis Day
Invisible and misunderstood: how it is to live with an autoimmune disease?
“I think the hardest part of having an autoimmune disease is spending any free time I have sick in bed due to using up what little energy I have […] I’m always mourning my old hobbies that I don’t have time or energy for anymore. Or friends that need me that I can’t be there for because of the exhaustion. People thinking I don’t make the time simply because I don’t care enough to, but really, I genuinely physically and mentally can’t without putting my body through even more than it’s already dealing with” [1].
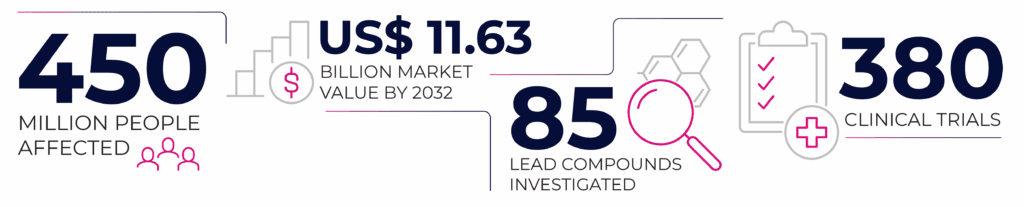
Approximately 450 million people worldwide, or 1 in 10, are suffering from symptoms of an autoimmune or autoinflammatory arthritis. Often misunderstood, misdiagnosed, labeled as a “mystery disease” and dismissed for years before a proper medical determination is made. This in turn takes toll on quality of life, relationships and cost of healthcare. It is then very important to promote education, advocacy, and therapeutic advances for those impacted by autoimmune and autoinflammatory arthritis. The goal is to accelerate detection and treatment, enhance outcomes and quality of life, empower patient involvement, and dispel misconceptions about inflammatory arthritis.
Pursuit for the perfect medication: understanding the mechanisms behind autoimmune disorders
While there may be no permanent cure for autoimmune diseases, a wide range of therapeutic options are currently available to help alleviate symptoms and enhance patients’ quality of life. Although the key elements of autoimmunity have been identified, the complex molecular pathways underlying the disease remain not fully understood. As a result, no current therapeutic option is entirely effective or free from side effects. Suppressing the immune system – which normally defends the body but malfunctions in autoimmune diseases – can, for example, increase a person’s susceptibility to infections. Current therapeutic options include conventional immunosuppressors, such as methotrexate, sulfasalazine or hydroxychloroquine. There are multiple biologic and small molecule therapeutics available, which target specific cytokines including TNF (adalimumab, infliximab, etanercept), IL-6 (tocilizumab), IL-1 (anakinra). Other medications work on immune cells, for example, blocking T-cell activation (abatacept) or B-cell depletion (rituximab). More recently, patients can receive JAK inhibitors such as tofacitinib, rinvoq or olumiant. The therapeutic field remains highly competitive and active, with ongoing efforts to develop more effective drugs with fewer side effects. Currently, there are around 85 different drug candidates targeting an overreactive immune system in clinical development, studied in more than 380 clinical trials [2].
Great undertaking requires reliable tools: ChemPartner offers precise and translatable preclinical autoimmune arthritis models
Preclinical animal studies are crucial in understanding of mechanism of action of an investigated compound, especially in such complex diseases as autoimmune arthritis. They are critical to establish new drug potency, efficacy and safety before progressing into clinical trials. One of the most useful models used in preclinical safety in rodents are collagen induced arthritis (CIA) and adjuvant induced arthritis (AIA).
From cartilage to clarity: decoding human RA through collagen-induced arthritis
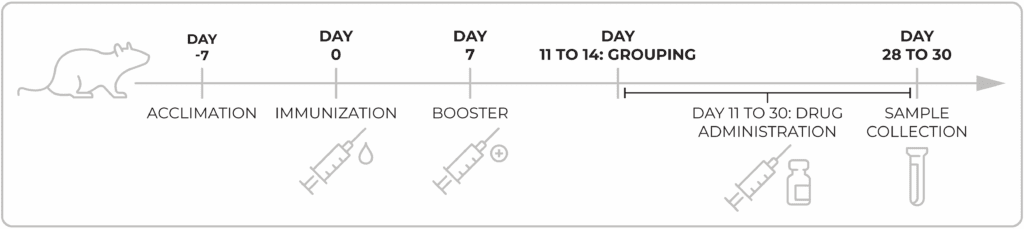
In collagen induced arthritis, the immune response is triggered by a joint antigen, usually by intra-dermal injection of collagen type II, which induces production of auto antibodies towards the collagen and the tissue itself. CIA is often considered the gold standard in vivo model for rheumatoid arthritis and shares multiple pathological and immunological features with human disease. CIA is T helper cell mediated and induces both T helper Th1 and Th17 responses. Rats usually develop severe polyarthritis two weeks after immunization, with swelling of the limbs, which persists for a few weeks, then decreases, before returning as a chronic disease eventually resulting with limb malformations. Rat models are used most often to test the drug candidates in that particular late, chronic stage of arthritis.
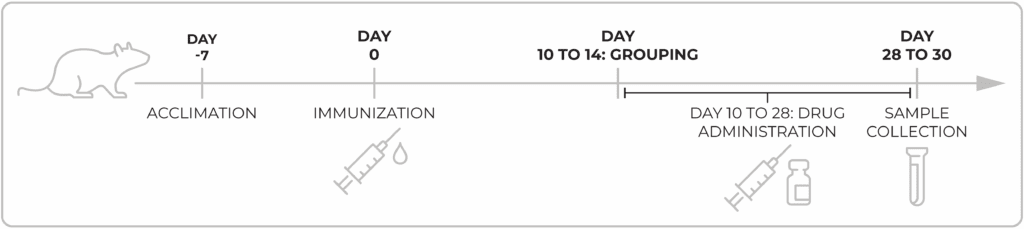
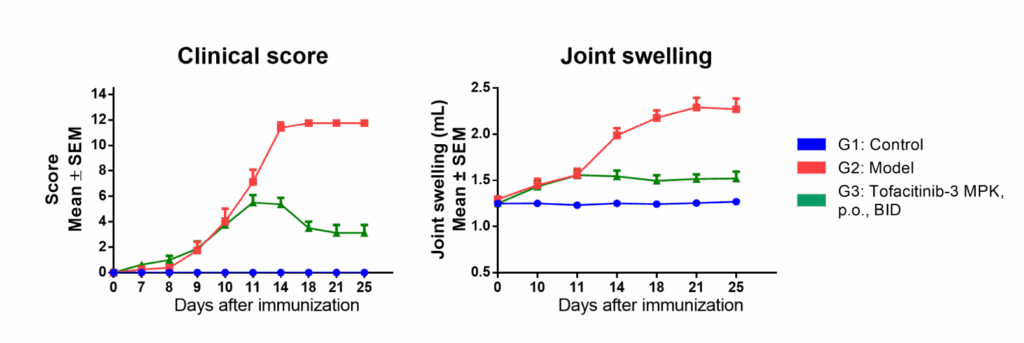
Chempartner Type-II collagen induced arthritis (CIA) model in rats
Model evaluation
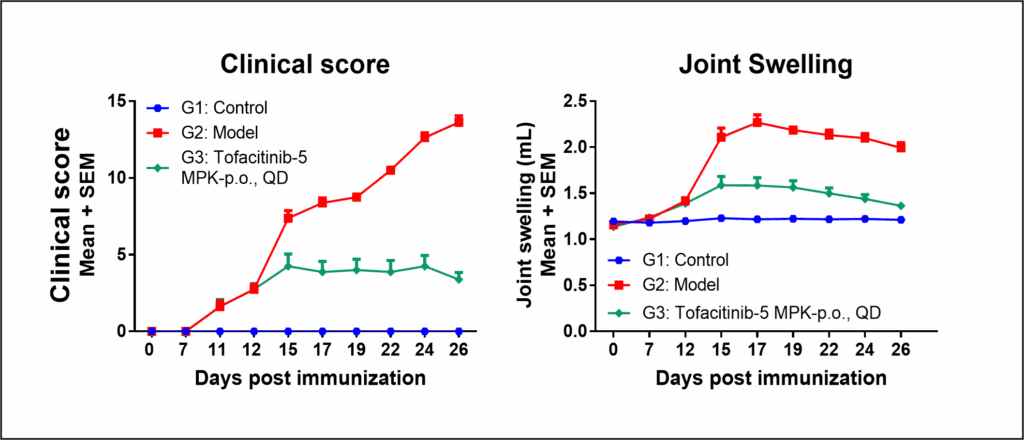
Both clinical score and joint swelling show model relevancy for RA and responsiveness to tofacitinib, a Janus kinase (JAK) inhibitor.
The model changes are also observable directly during the anatomical and histopathological evaluation below (CPD = tofacitinib).
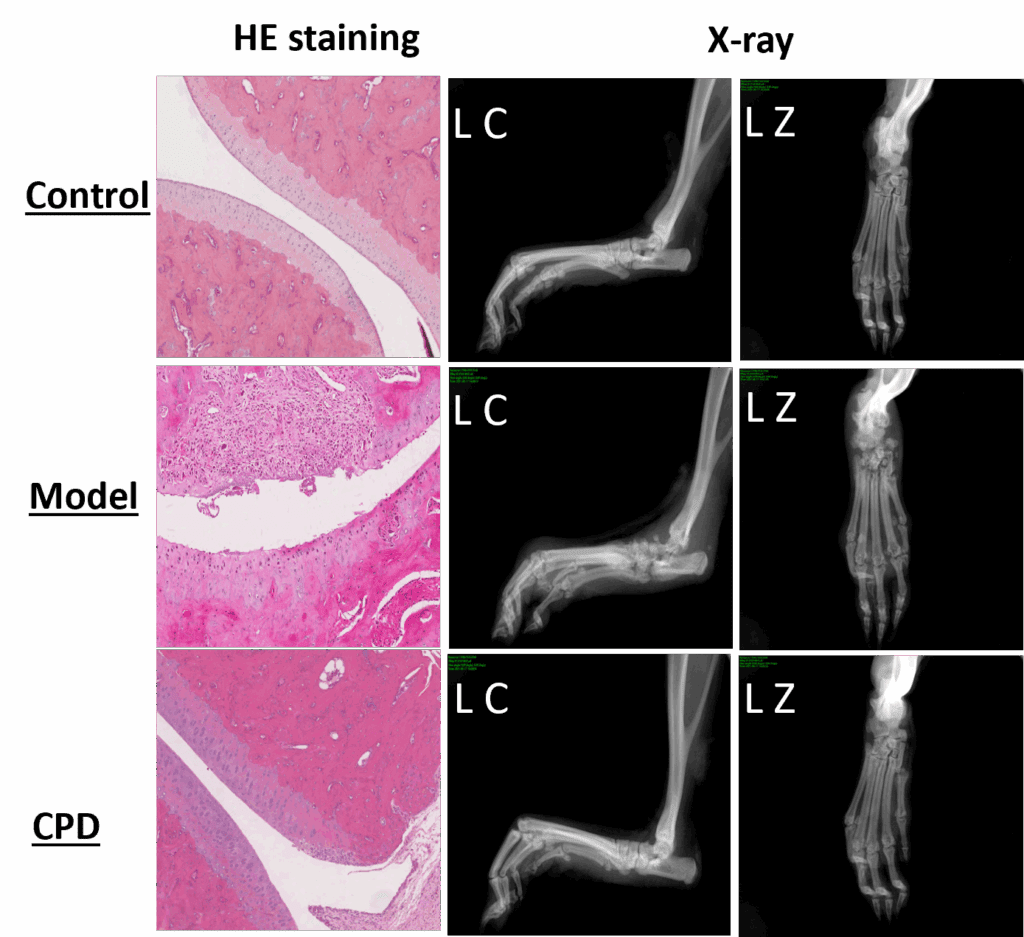
Model application for compound testing
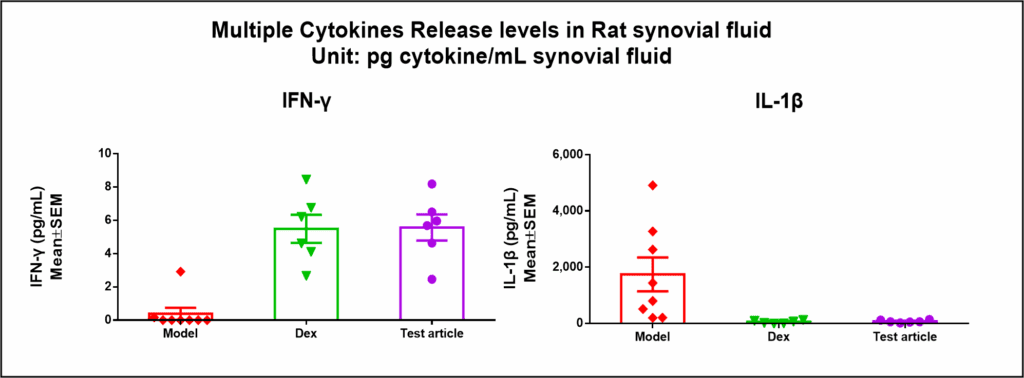
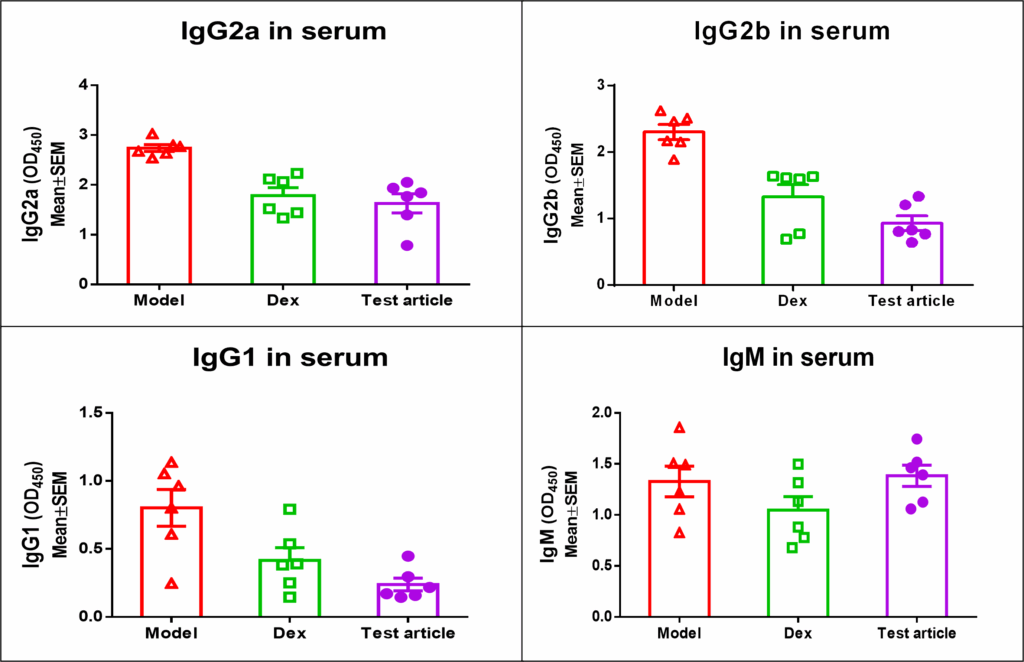
Rat CIA model was used to test an experimental compound. Dexamethasone was used as a control anti-inflammatory agent. Below are the results from cytokine and IgG panels.
ChemPartner collagen induced arthritis in DBA/1 mice model
Similarly to rats, the response is induced by immunization with heterologous type II collagen but the clinical signs of arthritis appear between 21–25th day and can also be additionally boosted after the initial inoculation. The model features inflammation of the synovial fluid, cartilage damage and bone erosion similar to human RA. [3]
Model evaluation
Both clinical score and joint swelling show model relevancy for RA and responsiveness to dexamethasone, an anti-inflammatory agent.
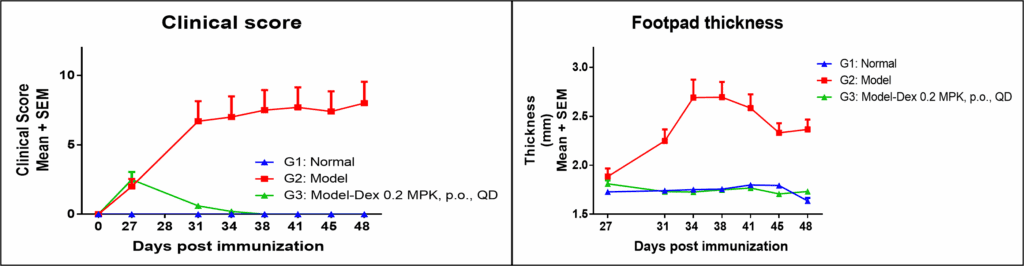
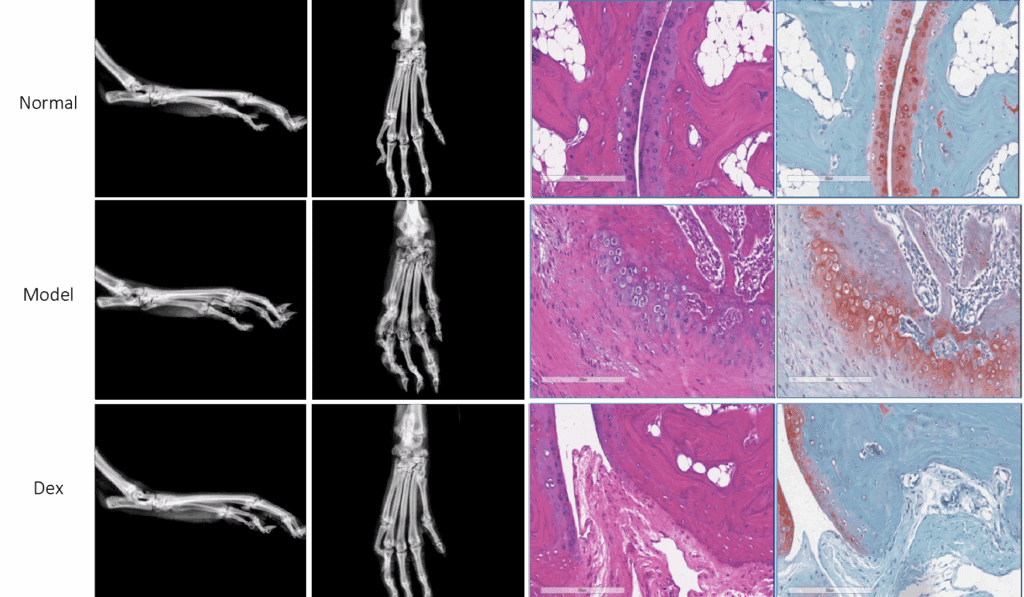
These changes can be directly observable using anatomical and histopathology observation
Adjuvant-induced arthritis: a rapid-onset model for chronic joint inflammation
Adjuvant induced arthritis in rats is induced by intradermal injection of complete Freund’s adjuvant (CFA) at the base of the tail or hind paw region. AIA model features a rapid onset and progression to polyarticular inflammation, where only after 10–14 days, the symptoms of arthritis develop. The observable features include permanent joint malformations, including ankylosis and share common symptoms with human RA such as joint swelling, lymphocyte infiltration and cartilage degradation. In rats with AIA, activated T cells can be detected in the inflamed joints. [3]
Adjuvant induced arthritis (AIA) in Rats
Model evaluation
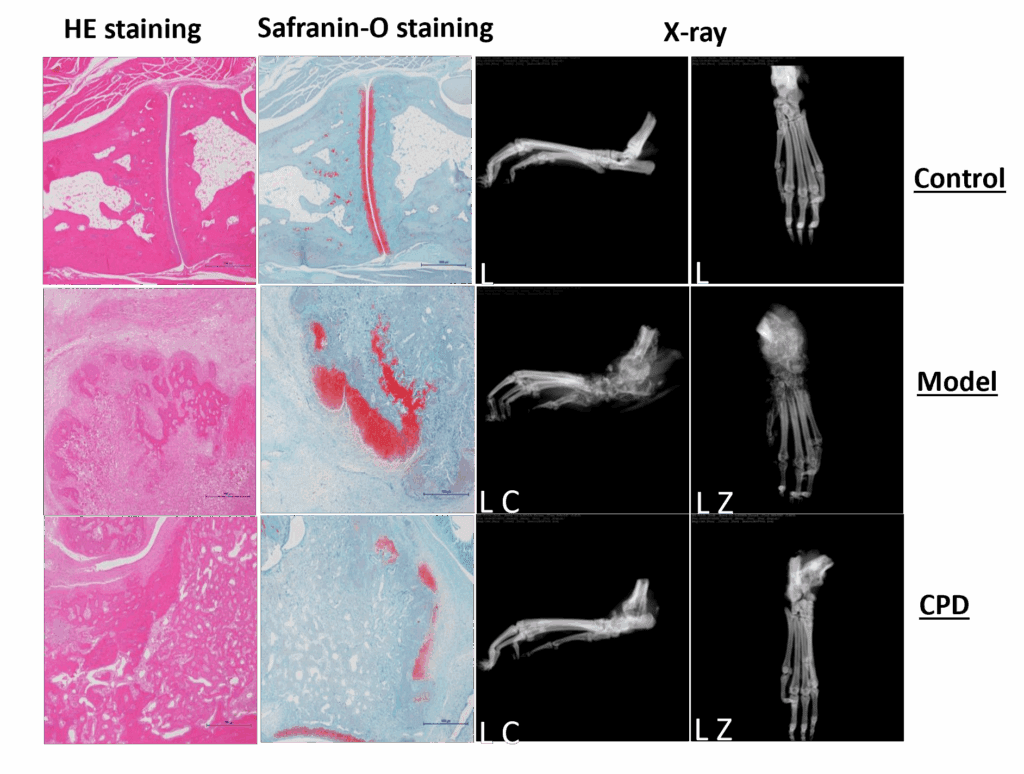
Model anatomy and histopathological analysis confirm model adequacy for preclinical RA compound testing.
Also, both clinical score and joint swelling show model relevancy for RA and responsiveness to tofacitinib, a Janus kinase (JAK) inhibitor.
Turning research into relief: advancing arthritis research with preclinical models
In the pursuit of more effective therapies for autoimmune and autoinflammatory arthritis, the journey begins long before a drug reaches the patient—it starts in the lab. At ChemPartner, we recognize the vital role preclinical models play in driving innovation, shaping our understanding of complex disease mechanisms, and enabling the development of safer, more effective therapies. As we honor AiArthritis Day 2025, we stand with the global community in raising awareness, advocating for patients, and contributing scientific excellence to the fight against these often invisible and life-altering diseases. With robust, translatable models, ChemPartner is proud to be part of the solution – because every breakthrough brings us one step closer to hope, healing, and a better quality of life for patients.
References
[1] u/justxpeachyii, Tough days living with autoimmune disease, Reddit, 2024, LINK
[2] Bhandari, M., Smith, J. F., Capra, E., & Yang, G. (2025). The race to reset autoimmune diseases. https://doi.org/10.1038/d41573-025-00085-z
[3] Choudhary, N., Bhatt, L. K., & Prabhavalkar, K. S. (2018). Experimental animal models for rheumatoid arthritis. Immunopharmacology and immunotoxicology, 40(3), 193–200. https://doi.org/10.1080/08923973.2018.1434793
ResearchAndMarkets.com. (2024, December 17). Autoimmune disease diagnostics market (By disease, test types, country analysis), key company profiles, strategy, financial insights, recent developments – Forecast to 2032. Business Wire. https://www.researchandmarkets.com/r/qyljzd