Mouse Clinical Trial in PDXs
Objective: to evaluate drug efficacy across 17 colorectal PDXs
Pre-defined Clinical Endpoints
- Tumor Progression
- A tumor volume change >73% by the end of treatement
- Tumor Stasis
- A tumor volume change between -66% and 73% by the end of treatment
- Tumor Regression
- A tumor volume change ≤ -66% by the end of treatment
- Complete Tumor Regression
- Non-palpable tumors
%TV = ((TV end of treatment – TV start of treatment)/TV start of treatment) x 100
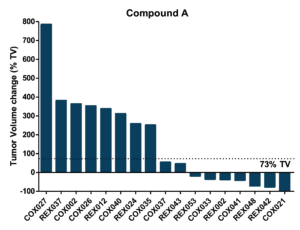
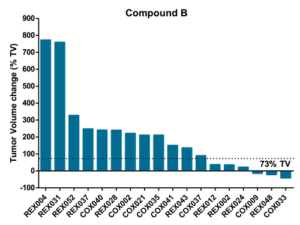
Efficacy and Biomarker Assessment in PDXs
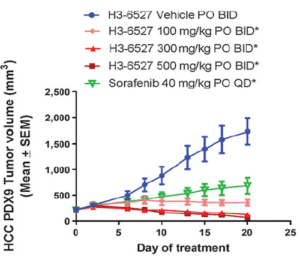
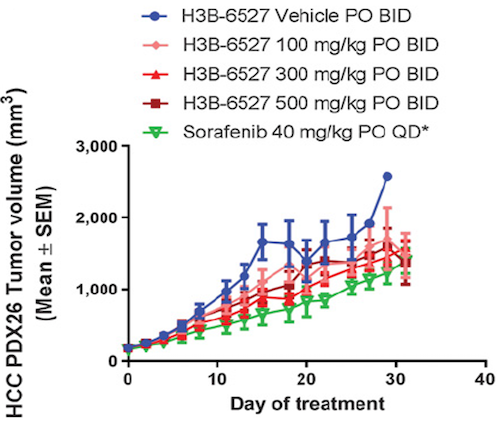
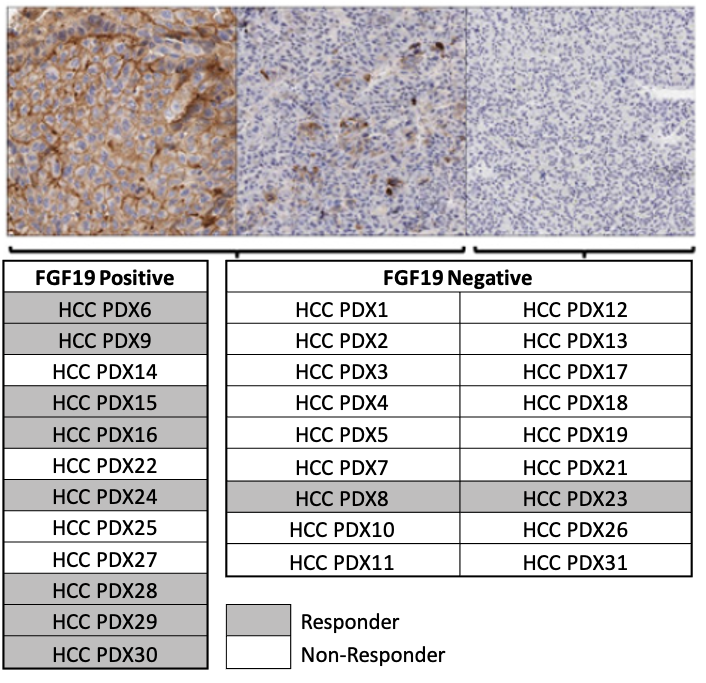
Cancer Res; 2017, 77(24);6999-7013
- Efficacy of FGFR4 inhibitor in 30 HCC PDX models, and FGF19 IHC were all performed at ChemPartner
- FGF19 expression level correlates well with drug efficacy, implicating that FGF19 overexpression is a predictive biomarker for FGFRi response.

