Trastuzumab-DM1 (T-DM1; biosimilar of Kadcyla®) was synthesized and was tested in an in vitro efficacy assay employing an / xenograft model.
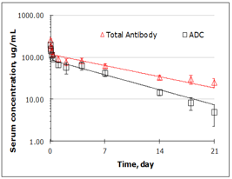
The following results demonstrate the in-house T-DM1 has comparable tumor inhibition activity and / PK profiles comparing to reported data of Kadcyla.
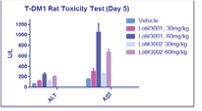
Acute toxicity of T-DM1 was assessed. Results showed tested ADC products are well tolerated at the tested dosages
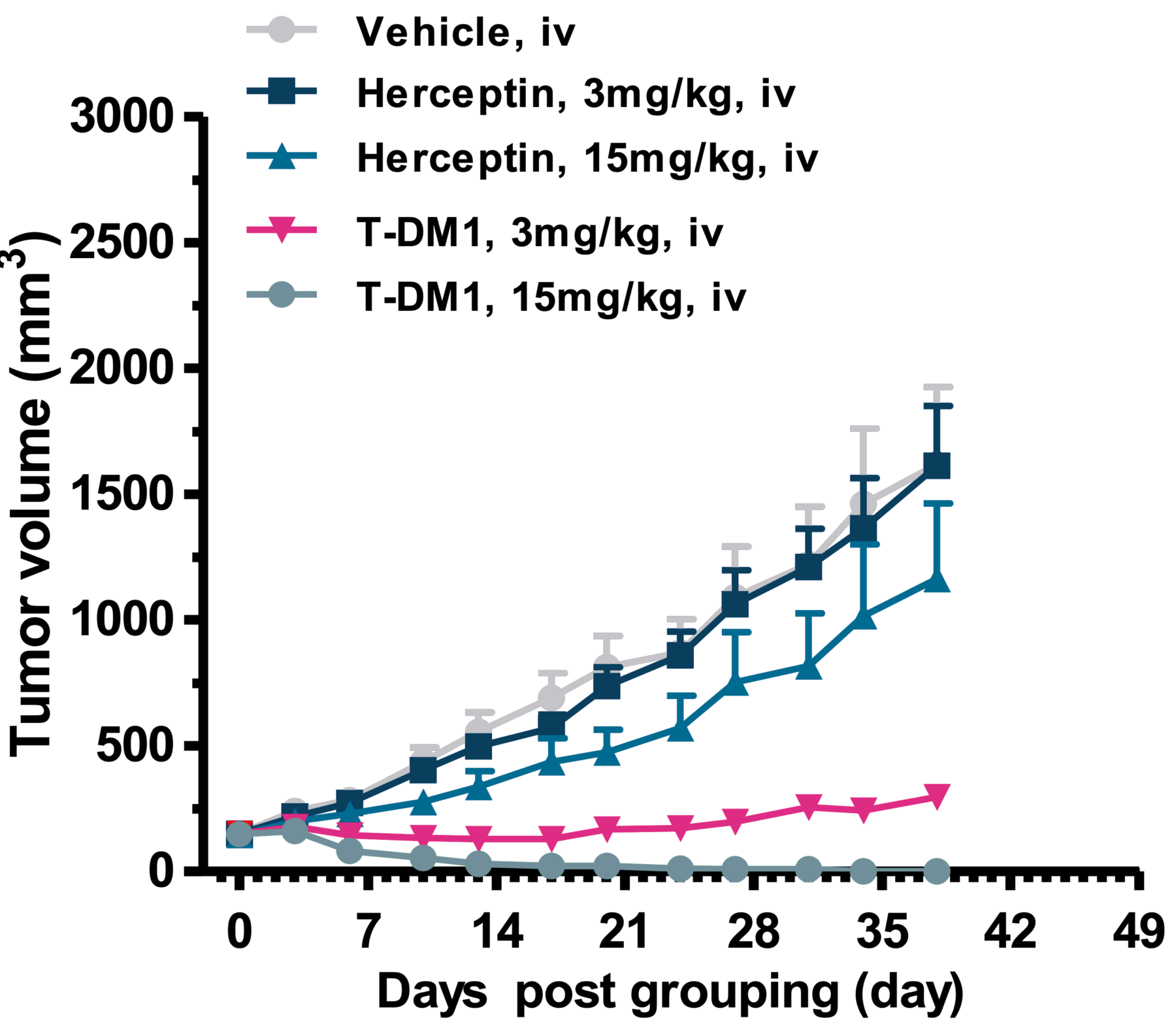
In vivo efficacy results from T-DM1 case study using NCl-N87 gastric cancer xenograft model. Data showed full regression of tumor after treatment with 15mg/kg of ADC.