This blog series presents links to new literature on topics of Medicinal and Organic Chemistry.
It is derived from surveys of the Table of Contents for the Journal of Medicinal Chemistry, ACS Medicinal Chemistry Letters, Journal of Organic Chemistry, Journal of the American Chemical Society, Organic Letters, Organic Process Research and Development, Bioconjugate Chemistry, ACS Chemical Biology, Accounts of Chemical Research, Chemical Reviews ,and others with overviews provided by the blog author.
Recent advances in metal-catalysed asymmetric sigmatropic rearrangements
Authors: Yangbin Liu, Xiaohua Liu, and Xiaoming Feng
Abstract
Asymmetric sigmatropic rearrangement is a powerful organic transformation via substrate-reorganization to efficiently increase molecular complexity from readily accessible starting materials. In particular, a high level of diastereo- and enantioselectivity can be readily accessed through well-defined and predictable transition states in [3,3], [2,3]-sigmatropic rearrangements, which have been widely applied in the synthesis of various chiral building blocks, natural products, and pharmaceuticals. In recent years, catalytic asymmetric sigmatropic rearrangements involving chiral metal complexes to induce stereocontrol have been intensively studied. This review presents an overview of metal-catalysed enantioselective versions of sigmatropic rearrangements in the past two decades, mainly focusing on [3,3], [2,3], and [1,3]-rearrangements, to show the development of substrate design, new catalyst exploitation, and novel cascade processes. In addition, their application in the asymmetric synthesis of complex natural products is also exemplified.
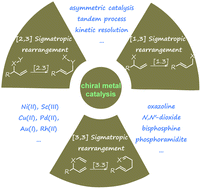
Overview
The recent literature of these rearrangements is reviewed including asymmetric examples. This is a useful method to make quaternary carbon centers with a C=Y function nearby.
Advancing New Chemical Modalities into Clinical Studies
Authors: Maria-Jesus Blanco, Kevin M. Gardinier, Mark N. Namchuk
Abstract
Drug discovery and development has experienced an incredible paradigm shift in the past two decades. What once was considered a predominant R&D landscape of small molecules within a prescribed properties and mechanism space now includes an innovative wave of new chemical modalities. Scientists in the pharmaceutical industry can now strategize across a variety of modalities to find the best option to modulate a given target and provide treatment for a specific disease. We have witnessed a remarkable change not only in molecular design but also in creative approaches to drug delivery that have enabled advancement of novel modalities to clinical studies. In this Microperspective, we evaluate the critical differences between traditional small molecules and beyond rule of 5 compounds, peptides, oligonucleotides, and biologics for advancing into development, particularly their pharmacokinetic profiles and drug delivery strategies.
Overview
From M.-J. Blanco et al.. This short review covers very recent literature with comparative perspective on development considerations for small molecules vs. bRo5 compounds like PROTACs, peptides, macrocycles, ASOs, MABs and ADCs. A nice job commenting on the issues posed by individual examples of this latter group is presented and how these challenges are approached. It is a little light on structures, but the references are there.
Co-Catalyzed Dual C5/C8–H Bond Functionalization of Imidazo[1,2-a]pyrazines with Disulfides and Grignard Reagents
Authors: Bo Zhang, Wensheng Zhang, Jiaoli Wang, Qiuan Wang, Nobuaki Kambe, Renhua Qiu
Abstract
An efficient difunctionalization at C5/C8 of imidazo[1,2-a]pyrazines has been developed using disulfides and Grignard reagents under cheap cobalt catalysis. This one-pot, two-step, three-component transformation is performed under mild conditions; various Grignard reagents (aryl and alkyl) and disulfides are tolerated. Mechanistic studies and control experiments demonstrate this reaction proceeded via an anionic intermediate.
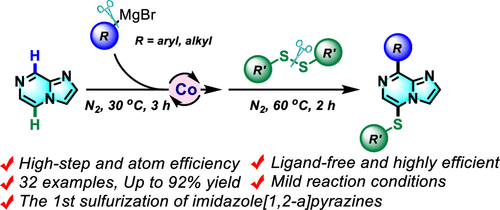

Overview
From Q. Wang and R. Qiu et al. Imidazo[1,2-a]pyrazines are valuable fused heterocycles in drug discovery. This method allows positions C5 and C8 to have Hs substituted for any Grignard-based carbon with PhS- on the opposite side. The PhS- group can be manipulated further in cross-coupling, oxidation, or even Raney Nickel-mediated removal (not exemplified).
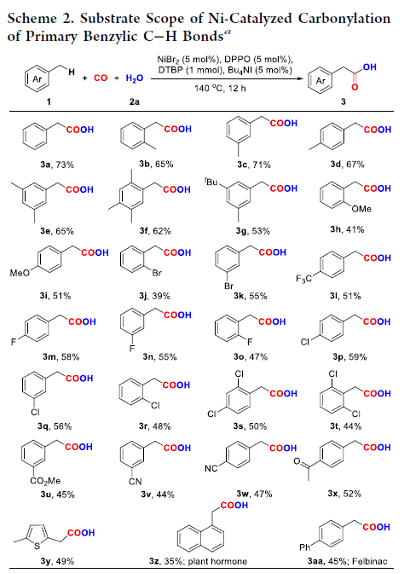
Nickel-Catalyzed Oxidative Carbonylation of Alkylarenes to Arylacetic Acids
Authors: Yongzheng Ding, Renbin Huang, Wei Zhang, Hanmin Huang
Abstract
A catalytic system combined with NiBr2 and diphenylphosphine oxide that enabled direct access to the valuable arylacetic acids from inexpensive alkylarenes and H2O via oxidative carbonylation was developed. Alkylarenes with primary and secondary benzylic C–H bonds were compatible with this method. Remarkably, the marketed drugs ibuprofen and diclofenac could be easily obtained by this procedure straightforwardly.
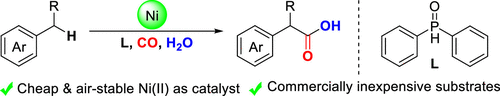

Overview
From H. Huang et al. Arylacetic acids are valuable synthesis targets that sometimes require multiple steps to access. Here is a one-step Ni-catalyzed approach using CO as the CO2H source. Based on the examples listed, this seems promising.

Orally Bioavailable Prodrugs of ψ-GSH: A Potential Treatment for Alzheimer’s Disease
Authors: Wei Xie, Bin Cao, Haizhou Zhu, Abbas Raza, Nicholas Juckel, Jiashu Xie, Rongrong Jiang, Robert Vince, Michael K. Lee, Swati S. More
Abstract
Addressing glycation-induced oxidative stress in Alzheimer’s disease (AD) is an emerging pharmacotherapeutic strategy. Restoration of the brain glyoxalase enzyme system that neutralizes reactive dicarbonyls is one such approach. Toward this end, we designed, synthesized, and evaluated a γ-glutamyl transpeptidase-resistant glyoxalase substrate, ψ-GSH. Although mechanistically successful, the oral efficacy of ψ-GSH appeared as an area in need of improvement. Herein, we describe our rationale for the creation of prodrugs that mask the labile sulfhydryl group. In vitro and in vivo stability studies identified promising prodrugs that could deliver pharmacologically relevant brain levels of ψ-GSH. When administered orally to a mouse model generated by the intracerebroventricular injection of Aβ1–42, the compounds conferred cognitive benefits. Biochemical and histological examination confirmed their effects on neuroinflammation and oxidative stress. Collectively, we have identified orally efficacious prodrugs of ψ-GSH that are able to restore brain glyoxalase activity and mitigate inflammatory and oxidative pathology associated with AD.

Overview
From S. S. More et al. This surveys several potential prodrugs of the a-CH2SH group which is relevant for a key lead molecule in Alzheimer’s disease research by restoring brain glyoxalase activity. Prodrugs which improve oral bioavailability and brain delivery were discovered that also gave cognition improvement POC.








