This blog series presents links to new literature on topics of Medicinal and Organic Chemistry.
It is derived from surveys of the Table of Contents for the Journal of Medicinal Chemistry, ACS Medicinal Chemistry Letters, Journal of Organic Chemistry, Journal of the American Chemical Society, Organic Letters, Organic Process Research and Development, Bioconjugate Chemistry, ACS Chemical Biology, Accounts of Chemical Research, Chemical Reviews ,and others with overviews provided by the blog author.
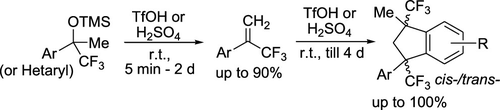
Synthesis of α-(Trifluoromethyl)styrenes and 1,3-Di(trifluoromethyl)indanes via Electrophilic Activation of TMS Ethers of (Trifluoromethyl)benzyl Alcohols in Brønsted Acids
Authors: Olesya V. Khoroshilova, Irina A. Boyarskaya, Aleksander V. Vasilyev
Abstract
TMS ethers of CF3-benzyl alcohols (and their heterocyclic analogues) undergo elimination of TMSOH molecule with the formation of α-(trifluoromethyl)styrenes in yields of up to 90% under the action of strong Brønsted acids TfOH or H2SO4. Under the acidic conditions upon prolongation of the reaction, the α-(trifluoromethyl)styrenes are further dimerized into cis-/trans-1,3-di(trifluoromethyl)indanes in yields of up to 100%. Plausible reaction mechanisms of these electrophilic transformations including the intermediate formation of CF3-benzyl carbocations are discussed.
Overview
From O. V. Khoroshilova, A. V. Vasilyev et al. This synthesis of geminal aryl-CF3-vinyl from the TMSCF3 adduct of ketones is a promising simple entry into this valuable group. Some interesting reactivity of this vinyl under acidic media is also disclosed including the following:
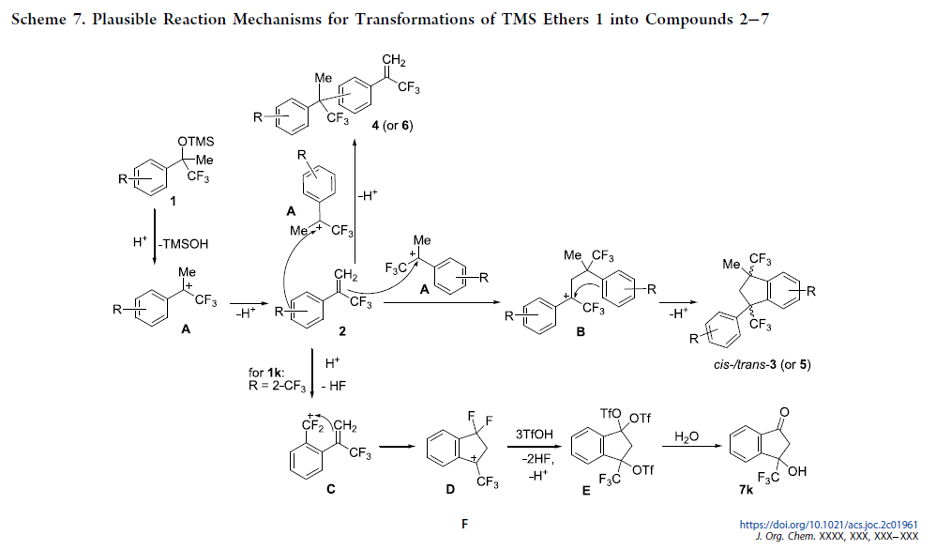
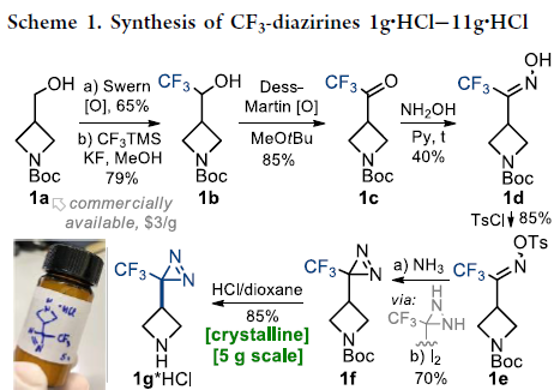
Fluorinated Aliphatic Diazirines: Preparation, Characterization, and Model Photolabeling Studies
Authors: Yurii Kornii, Oleg Shablykin, Taras Tarasiuk, Oleksandr Stepaniuk, Vitaliy Matvienko, Danylo Aloshyn, Nataliia Zahorodniuk, Iryna V. Sadkova, Pavel K. Mykhailiuk
Abstract
The previously unknown difluoromethyl diazirines and the previously neglected trifluoromethyl-aliphatic diazirines were synthesized and characterized. Model photolabeling experiments and biological studies showed that these compounds could indeed be used as photoaffinity labels.
Overview
From Y. Kornii, P. K. Mykhailiuk et al. This work shows the first general synthesis of CF3- and CHF2-diazirines on cyclic amines with evidence of their usefulness as biological study photoaffinity labels when incorporated into drug molecules.
In case you are interested in familial comparisons of reactivity, this information is in the SI:
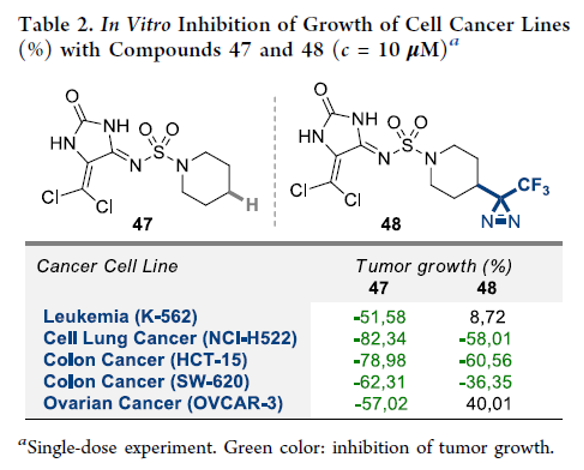
A Safer Reduction of Carboxylic Acids with Titanium Catalysis
Authors: P. Veeraraghavan Ramachandran, Abdulkhaliq A. Alawaed, Henry J. Hamann
Abstract
Ammonia-borane, shown previously to react with carboxylic acids under reflux to form primary amides, reduces acids to alcohols at room temperature in the presence of catalytic TiCl4. The process, which is tolerant of a variety of potentially reactive functional groups, including N-protected amino acids, can be employed for the selective reduction of acids in the presence of amides, nitriles and, to some extent, esters. Aliphatic acids can be selectively reduced in the presence of aromatic acids.

Overview
From P. V. Ramachandran et al. Here is a selective mild CO2H reduction method. Personally, I like CDI/THF reflux followed by NaBH4. This may have advantages with scale economy.
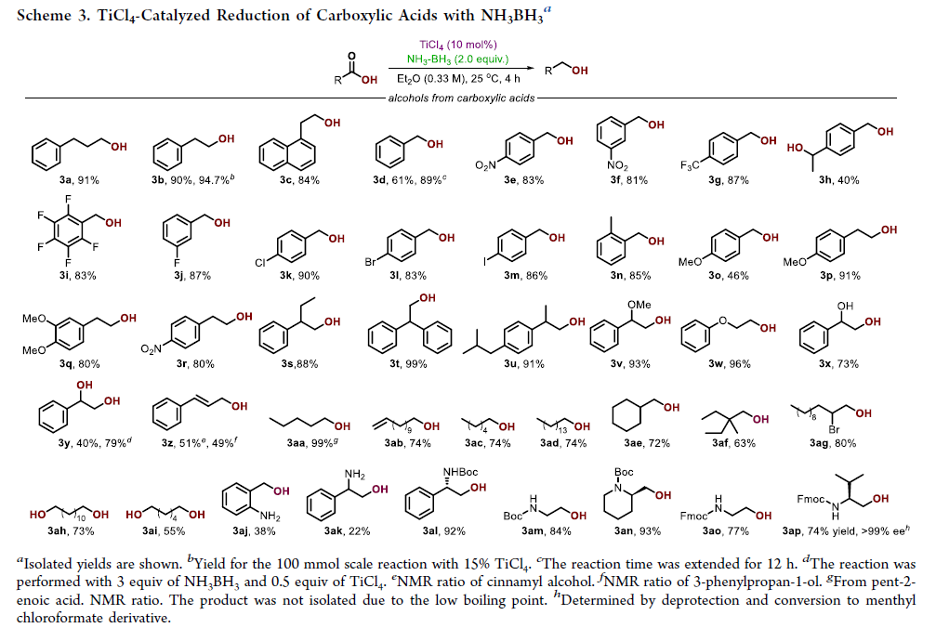

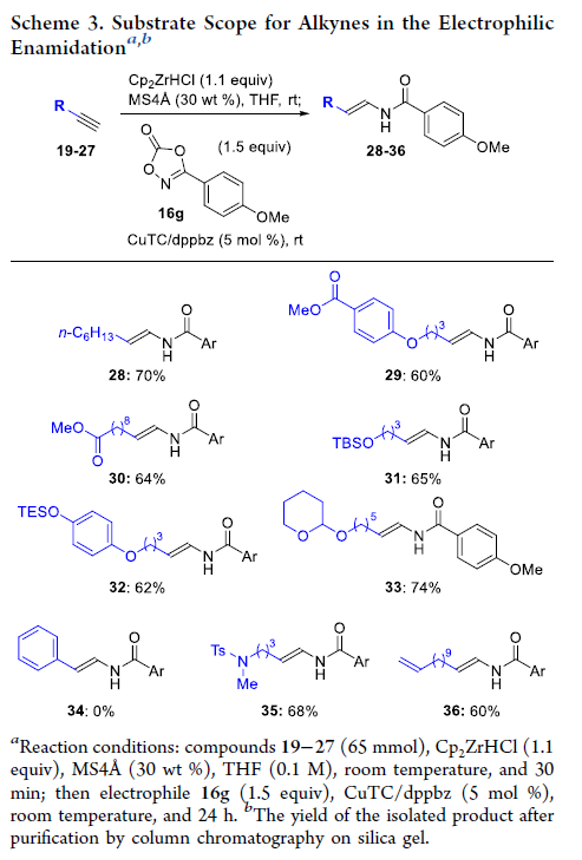
Copper-Catalyzed Electrophilic Enamidation Using Dioxazolones through Hydrozirconation of Alkynes
Authors: Shona Banjo, Keisuke Nakata, Eiko Nakasuji, Soichiro Yasui, Noritaka Chida, Takaaki Sato
Abstract
A copper-catalyzed electrophilic enamidation starting from alkynes is reported. Hydrozirconation of an alkyne with the Schwartz reagent forms a vinyl zirconium intermediate, which directly undergoes a copper-catalyzed electrophilic enamidation with dioxazolones. High functional group tolerance of hydrozirconation enables the use of functionalized alkynes including esters. The developed conditions are successfully applied to syntheses of partial structures found in biologically active natural products.

Overview
From S. Banjo, T. Sato et al. The enamide is a functionality to keep your eye on. Here is one new synthesis.

6-Thioguanosine Monophosphate Prodrugs Display Enhanced Performance against Thiopurine-Resistant Leukemia and Breast Cancer Cells
Authors: Sarah Moreno, Magdalena FicklIngo Bauer, Melanie Brunner, Anna Rázková, Dietmar Rieder, Isabel Delazer, Ronald Micura, Alexandra Lusser
Abstract
Thiopurines are in widespread clinical use for the treatment of immunological disorders and certain cancers. However, treatment failure due to resistance or adverse drug reactions are common, asking for new therapeutic strategies. We investigated the potential of 6-thioguanosine monophosphate (6sGMP) prodrugs to overcome resistance to 6-thioguanine. We successfully developed synthetic routes toward diverse 6sGMP prodrugs, tested their proliferation inhibitory potential in different cell lines, and examined their mode of action. Our results show that 4-acetyloxybenzyl- and cycloSaligenyl-derivatized 6sGMP prodrugs are effective antiproliferative compounds in cells that are resistant to thiopurines. We find that resistance is related to the expression of salvage pathway enzyme HGPRT. Using TUC-seq DUAL, we demonstrate the intracellular conversion of 6sGMP prodrugs into bioactive 6sGTPs. Thus, our study offers a promising strategy for thiopurine therapy by using 6sGMP prodrugs, and it suggests TUC-seq DUAL as a simple and fast method to measure the success of thiopurine therapy.
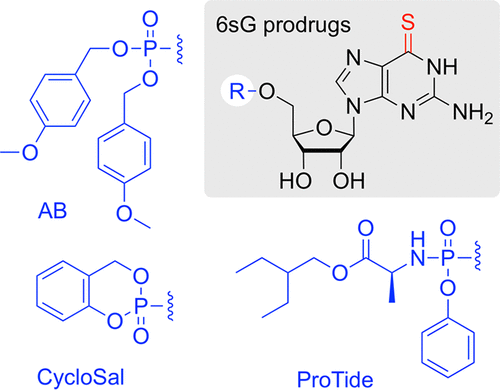

Overview
From S. Moreno, R. Micura, A. Lusser et al. For antiproliferative applications, 6-Thiophosphine prodrugs AB, and CycloSal work but ProTide does not, which is interesting since the ProTide seems preferred in all major antiviral drugs.







