This blog series presents links to new literature on topics of Medicinal and Organic Chemistry.
It is derived from surveys of the Table of Contents for the Journal of Medicinal Chemistry, ACS Medicinal Chemistry Letters, Journal of Organic Chemistry, Journal of the American Chemical Society, Organic Letters, Organic Process Research and Development, Bioconjugate Chemistry, ACS Chemical Biology, Accounts of Chemical Research, Chemical Reviews ,and others with overviews provided by the blog author.
Microwave-Assisted, Metal- and Azide-Free Synthesis of Functionalized Heteroaryl-1,2,3-triazoles via Oxidative Cyclization of N-Tosylhydrazones and Anilines
Authors: Zachary Z. Gulledge, Damian P. Duda, David A. Dixon, Jesse D. Carrick
Abstract
As the search for competent soft-Lewis basic complexants for separations continues to evolve toward identification of a chemoselective moiety for speciation of the minor actinides from the electronically similar lanthanides, synthetic methods must congruently evolve. Synthetic options to convergently construct unsymmetric heteroaryl donor complexants incorporating a 1,2,3-triazole from accessible starting materials for evaluation in separation assays necessitated the development of the described methodology. In this report, metal- and azide-free synthesis of diversely functionalized pyridyl-1,2,3-triazole derivatives facilitated by microwave irradiation was leveraged to prepare a novel class of tridentate ligands. The described work negates the incorporation of thermally sensitive and toxic organoazides by using N-tosylhydrazones and anilines as viable synthetic equivalents in an efficient 12 min reaction time. Adaptation to alternative synthons useful for drug discovery was also realized. Method discovery, optimization, N-tosylhydrazone and aniline substrate scope, as well as a preliminary mechanistic hypotheses supported by DFT calculations are reported herein.
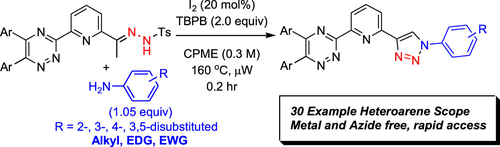
Overview
From the J. D. Carrick group. This is topical considering the new Nobel Prize announcement is all about the usefulness of being able to make triazoles with click chemistry in biological media. This is a new way to make triazoles without azides. Though not likely possible in a biological system since it requires heating at 160 oC, it will be useful since the starting materials derive from arylmethylketones and anilines. It would be interesting to see if it can go beyond the methyl ketone so substituents at C5 of the triazole would be possible.
Multicomponent Macrocyclic IL-17a Modifier
Authors: Eman Abdelraheem, Max Lubberink, Wenja Wang, Jingyao Li, Atilio Reyes Romero, Robin van der Straat, Xiaochen Du, Matthew Groves, and Alexander Dömling
Abstract
IL-17a is a major inflammation target, with several approved antibodies in clinical use. Small-molecule IL-17a antagonists are an emerging hot topic, with the recent advancement of three compounds into clinical trials. Here, we describe the design, discovery, synthesis, and screening of macrocyclic compounds that bind to IL-17a. We found that all currently described IL-17a modifiers belong to the same pharmacophore model, likely resulting in a similar receptor binding mode on IL-17a. A pipeline of pharmacophore analysis, virtual screening, resynthesis, and protein biophysics resulted in a potent IL-17a macrocyclic modifier.
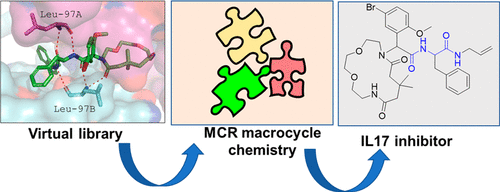
Overview
From the A. Domling group. Research into macrocyclic IL-17a inhibitors was driven by recent entry of 3 similar macrocycles into clinical trials. From a simple library synthesis of 1000 compounds, new leads were discovered and a structural binding model was proposed.
Enantioselective Synthesis of Atropisomers via Vinylidene ortho-Quinone Methides (VQMs)
Authors: Wenling Qin, Yidong Liu, and Hailong Yan
Abstract
Conspectus
Atropisomers, arising from conformational restriction, are inherently chiral due to the intersecting dissymmetric planes. Since there are numerous applications of enantiopure atropisomers in catalyst design, drug discovery, and material science, the asymmetric preparation of these highly prized molecules has become a flourishing field in synthetic chemistry. A number of catalysts, synthetic procedures, and novel concepts have been developed for the manufacture of the atropisomeric molecules. However, due to the intrinsic properties of different types of atropisomers featuring biaryl, hetero-biaryl, or non-biaryl architectures, only very few methods pass the rigorous inspection and are considered generally applicable. The development of a broadly applicable synthetic strategy for various atropisomers is a challenge. In this Account, we summarize our recent studies on the enantioselective synthesis of atropisomers using the vinylidene ortho-quinone methides (VQMs) as pluripotent intermediates.
The most appealing features of VQMs are the disturbed aromaticity and axial chirality of the allene fragment. At the outset, the applications of VQMs in organic synthesis have been neglected due to their principal liabilities: ephemeral nature, extraordinary reactivity, and multireaction sites. The domestication of this transient intermediate was demonstrated by in situ catalytic asymmetric generation of VQMs, and the reactivity and selectivity were fully explored by judiciously modifying precursors and tuning catalytic systems. A variety of axially chiral heterocycles were achieved through five-, six-, seven- and nine-membered ring formation of VQM intermediates with different kinds of branched nucleophilic functional groups. The axially chiral C–N axis could be constructed from VQM intermediates via N-annulation or desymmetrization of preformed C–N scaffolds. We take advantage of the high electrophilicity of VQMs toward a series of sulfur and carbon based nucleophiles leading to atropisomeric vinyl arenes. Furthermore, chiral helical compounds were realized by cycloaddition or consecutive annulation of VQM intermediates. These achievements demonstrated that the VQMs could work as a nuclear parent for the collective synthesis of distinct and complex optically active atropisomers. Recently, we have realized the isolation and structural characterization of the elusive VQMs, which were questioned as putative intermediates for decades. The successful isolation of VQMs provided direct evidence for their existence and an unprecedented opportunity to directly investigate their reactivity. The good thermal stability and reserved reactivity of the isolated VQMs demonstrated their great potential as synthetic reagents and expanded the border of VQM chemistry.
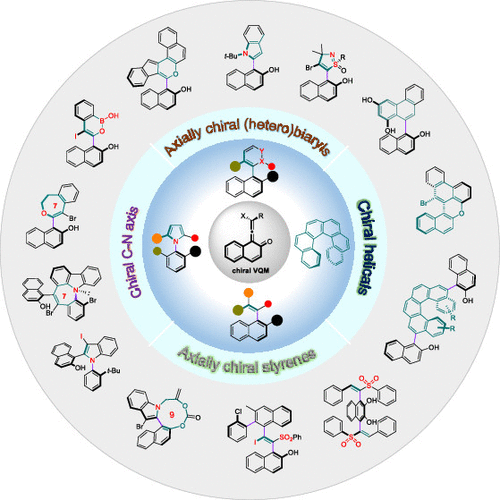
Overview
From the H. Yan group. A review of selective atropisomer synthesis. Atropisomers are another form of chirality that has created opportunities for novelty and selectivity in drug discovery projects but is challenged by the synthesis aspects. This review presents the vinylidene quinone methide (VQM) method for selective synthesis.
Catalyst-Tuned Electrophilic Chlorination of Diverse Aromatic Compounds with Sulfuryl Chloride and Regioselective Chlorination of Phenols with Organocatalysts
Authors: Erkan Ertürk and Tolga A. Yeşil
Abstract
In this work, we demonstrate that diverse aromatic compounds can be selectively chlorinated through the fine-tuning of the reactivity of sulfuryl chloride (SO2Cl2) by organocatalysts. Acetonitrile has been identified to activate SO2Cl2 most strongly, thus enabling even chlorination of p-xylene with high yields. 1,4-Dioxane effects chlorination of oxidation-labile aromatic compounds such as p-cresol and 2-naphthol with high yields, 95% and 85%, respectively. An array of potential catalysts has been screened for ortho– and para-selective chlorination of phenols. Thus, we found that acetonitrile, (S)-BINAPO (5 mol %), and diisopropyl ether (4.00 equiv) can catalyze the chlorination of phenols in a para-selective manner (with ≤4:96 o:p ratio), whereas Nagasawa’s bis-thiourea (1 mol %), phenyl boronic acid (5 mol %), and (S)-diphenylprolinol (1 mol %) exhibit high ortho selectivity [with ≤99:1 o:p ratio by (S)-diphenylprolinol].
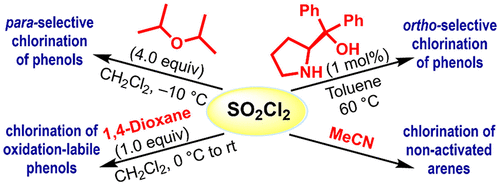
Overview
From the E. Erturk group. Use of SO2Cl2 and various simple additives to get selective ortho chlorination of phenols. Chlorination techniques typically favor the para-position but this approach shows ortho selectivity with a limited set of phenols and naphthols. It will be interesting to see the diversity that this system will tolerate.
N2-Selective β-Thioalkylation of Benzotriazoles with Alkenes
Authors:
Li-Li Zhu, Lifang Tian, Kunhui Sun, Yiwen Li, Guanglu Liu, Bin Cai, Hui Zhang, and Yahui Wang
Overview
From the Zhang and Wang group. Well, another triazole article, how topical still! Regioselective N2-alkylation of benzotriazoles and 3-substituted triazoles with vinyl groups. It places SPh on the other vinyl carbon. I wonder if Raney Ni can remove the sulfur so you effectively have a selective N2-alkylation onto a vinyl group.
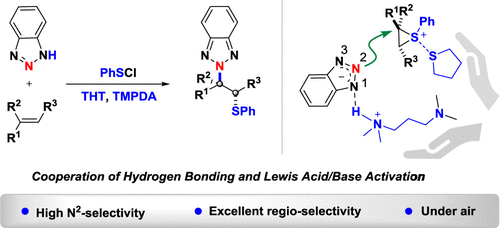
Overview
From the Zhang and Wang group. Well, another triazole article, how topical still! Regioselective N2-alkylation of benzotriazoles and 3-substituted triazoles with vinyl groups. It places SPh on the other vinyl carbon. I wonder if Raney Ni can remove the sulfur so you effectively have a selective N2-alkylation onto a vinyl group.








