Blog
From Neurons to Networks: High-Throughput Epilepsy Models for Translational Neuroscience
The Importance of Preclinical Models in Epilepsy Drug Discovery
Epilepsy is a chronic neurological condition affecting more than 50 million people worldwide and one of the most intensely investigated therapeutic areas in neuroscience. Despite many antiepileptic drugs existing on the market, there’s currently no effective cure for epilepsy, as all available medicines work by alleviating epilepsy symptoms only. With the current pharmacological treatment, often plagued with side effects, 30% of individuals develop drug resistant epilepsy. The demand for new, safer and more potent anticonvulsant treatments beyond symptom suppression requires robust experimental models in preclinical drug discovery and development. Early validation screening assays of drug efficacy and safety are in high demand for proper evaluation and assessment of translational potential of novel therapeutics that are safer, have higher specificity and disease-modifying potential. While multiple drug screening assays are available, most of them are currently low throughput and more suitable for mechanistic studies than for effective drug screening.

Multi-well, multi-electrode array (mwMEA) platforms enable high-throughput in vitro functional assays for modeling neurological diseases such as epilepsy.
Bridging In Vitro and In Vivo Models for Translational Epilepsy Research
Preclinical models are critical for early drug development, providing controlled environments to evaluate efficacy, safety, and pharmacokinetic profiles. Current models can mimic epilepsy from acute to chronic and simulate both induced and genetic-based epilepsy conditions. The combination of in vitro and in vivo models provides multilevel insight into disease mechanisms, from cellular and neuronal network levels up to translatable rodent-based models replicating various seizure types and epilepsy syndromes. An integrated approach using in vitro and in vivo models can significantly enhance the robustness and translatability of anticonvulsant drug screening. First, a high-throughput screening in an in vitro system can accelerate identification of promising leads and investigate their mechanism of action. Then, animal studies assess efficacy, toxicity, and pharmacokinetics within the context of a living organism.
In vitro models offer multiple advantages, including lowered cost, high reproducibility and reduced use of animals in research. Most of them have been developed based on neuronal cultures and brain slices, which have greatly contributed to our knowledge of seizures, and are considered the gold standard in neuroscience research. One of the methods for invoking a seizurogenic environment is to disrupt the extracellular media’s ion balance, since the ion gradient controls the excitability of neurons. For example, increasing the extracellular potassium content will increase the excitability of the neurons and induce seizure-like activity in cell cultures. Similarly, epileptic models can be established using ion channel inhibitors. For example, 4-Aminopyridine (4-AP) acts as a blocker of voltage-gated potassium channels, which prolongs action potentials and leads to increased neurotransmitter release. This in turn enhances synaptic transmission strength and contributes to convulsive effects in neuronal cultures.
Besides manipulating extracellular ion concentration or modulating voltage-gated potassium channels, seizurogenic conditions can be achieved by targeting excitatory or inhibitory pathways. For example, to emulate an acute seizure, pentylenetetrazole (PTZ) can act as a GABA antagonist, blocking inhibitory circuits, which causes epileptiform discharges in primary cultures of rodent neurons. This model is especially useful for drugs that enhance GABAergic inhibition. Some models use GABA-A receptor inhibition via picrotoxin to induce seizures characterized by hyperexcitability, which provide insights into inhibitory neurotransmission and are useful for mechanistic studies.
Epilepsy drug candidates can also be tested on genetic seizurogenic models, for example the Scn1a knockout mouse model, which serves as a model for Dravet syndrome (DS), characterized in humans by early-onset severe epilepsy, cognitive deficits and cases of sudden death. Mouse-derived in vitro systems are frequently employed to investigate DS pathophysiology via electrophysiological recordings and for in vivo validation, of which the difficulty to obtain a suitable cohort of DS mice is a bottle neck for carry out studies. Developing a robust high-throughput screening platform can significantly overcome this hurdle. [1][2]
ChemPartner Brings Reliable High Throughput Screening for Epilepsy
We have developed a multi-well, multi-electrode array (mwMEA) platform that enables high-throughput in vitro functional assays for modeling neurological diseases and accelerating drug screening. We established a chemically induced epilepsy models by applying traditional seizurogenic compounds with distinct mechanisms of action – picrotoxin (PTX), 4-aminopyridine (4-AP), and pentylenetetrazol (PTZ) – as well as elevated extracellular K⁺ concentrations, to rat hippocampal neurons cultured on mwMEA plates.
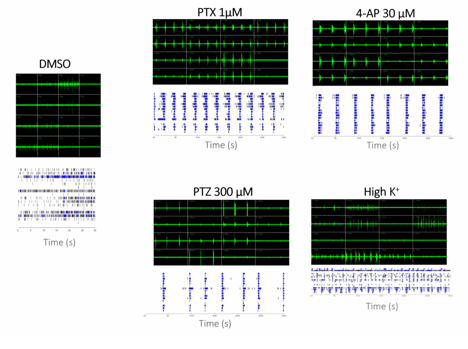
Increase of synchronized burst firing after treatment of various seizurogenic compounds.
The experimental setup utilized a multi-well microelectrode array (mwMEA) platform to record extracellular neuronal activities, with primary readouts including mean firing rate, burst duration, and network inter-burst interval (IBI) coefficient of variation (CV, assessing burst regularity and synchronization). Patch-clamp electrophysiology was employed to measure evoked action potentials and spike number evoked in response to current stimulation. For long-term monitoring in the genetic Dravet syndrome model, measurements encompassed mean spike frequency and spike number per burst. To test our models, we used Valproate (VPA) and Retigabine (RTG). VPA is an enhancer of GABAergic transmission and blocker of voltage-gated sodium channels. It exerts antiepileptic effects by increasing brain GABA levels, inhibiting GABA transaminase, and attenuating excitatory neurotransmission, making it effective against a broad spectrum of seizures. RTG is an activator of KCNQ channel and a modulator of GABA-A receptor. In epilepsy treatment, it reduces neuronal hyperexcitability by shifting KCNQ (Kv7) potassium channel activation to more negative potentials, increasing open channels at rest and dampening seizure propagation.
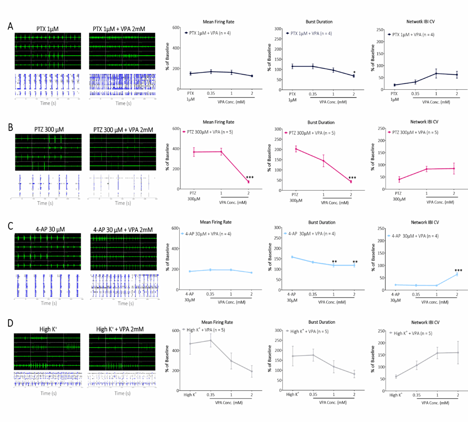
Our in mwMEA models’ validation shows increase of synchronized burst firing after treating cells with various seizurogenic compounds and the effect of Valproate at increasing concentration.

For the genetic seizurogenic model, we developed a DS model of severe epileptic encephalopathy characterized by recurrent and prolonged seizures. Primary neurons from DS mice were extracted at an early age and cultured long-term on mwMEA recording plates.
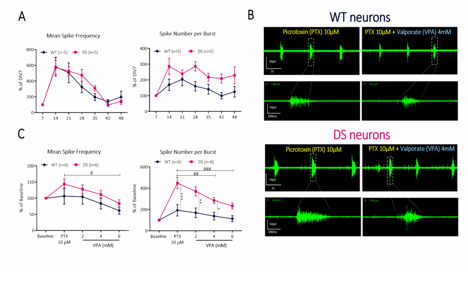
We evaluated Valproate in genetic Scn1a mutation cell models. Long-term supervision of excitability development on cortical neurons extracted from WT and SCN1A-mutant Dravet syndrome (DS) mouse pups (A). Comparative susceptibility of spike and burst firing in WT versus DS neurons after sequential exposure to pro-convulsant and anti-convulsant drugs (B, C).
Advancing Translational Epilepsy Research Through High-Throughput Innovation
ChemPartner’s integrated high throughput mwMEA platform bridges the gap between mechanistic insights and translational drug discovery, accelerating the identification and optimization of new anticonvulsant therapies. Our approach enables robust, reproducible, and high-content screening for next-generation antiepileptic drugs.
This blog post is only a teaser – for a deeper look at our experimental design, data insights, and case studies, visit our poster presentation at the Society for Neuroscience (SfN) Annual Meeting, titled “A High-Throughput In Vitro Functional Platform for Anti-Epileptic Drug Screening in Genetic and Chemically Induced Seizure Models”. Don’t miss the opportunity to connect with our scientists and explore how ChemPartner is transforming preclinical neuroscience research.
Bibliography
[1] Heydari, Y., Bozzi, Y. & Pavesi, L. Decoding Epileptic Seizures: Exploring In Vitro Approaches to Unravel Pathophysiology and Propel Future Therapeutic Breakthroughs. Biomedical Materials & Devices 2, 905–917 (2024). https://doi.org/10.1007/s44174-024-00158-4
[2] Shafieq, S., Ali, S. S., Sharma, N., & Sethi, V. A. (2025). Experimental Models for Anticonvulsant Research: A Comprehensive Review of In vivo and In vitro Approaches. International Journal of Newgen Research in Pharmacy & Healthcare, 3(1), 138–157. https://doi.org/10.61554/ijnrph.v3i1.2025.148