In the ongoing battle against cancer, antibody-drug conjugates (ADCs) shine as a beacon of hope. These resourceful molecules combine the precision of monoclonal antibodies with the potency of cytotoxic drugs, making them a formidable weapon against drug resistance and...
Search Results
Choosing the Right Antibody Generation Method: Phage Display, Hybridoma, or Single B Cell Cloning
There have been remarkable advancements in antibody generation technology over the years, offering scientists many techniques to generate antibodies for various applications ranging from discovery research to diagnostics and therapeutics. Three prominent methods for...
Equilibrium Dialysis vs. Ultracentrifugation for In Vitro Protein Binding Assays
In a modern drug discovery setting, in vitro protein binding assays are essential to assess the binding affinity and characteristics of a drug molecule with plasma proteins. Two commonly used methods for in vitro protein binding assays are equilibrium dialysis and...
In Vitro Safety Pharmacology: A Roadmap to Safer and More Effective Drugs
In vitro safety pharmacology profiling plays a crucial role in the drug development process by providing essential information about the potential safety and efficacy of new drug candidates. This approach involves conducting experiments in controlled laboratory...
3 Reasons to Introduce ADME Assays ASAP in Drug Discovery
Modern Drug Discovery Workflows There are several reasons why it is important to introduce in vitro absorption, distribution, metabolism, and excretion (ADME) assays as early as possible into a modern drug discovery workflow, especially for biotech companies. Here are...
Unlocking Success: Key Factors to Consider When Choosing the Right CDMO/CRO Partner
In order to stay competitive in the biopharmaceutical industry, companies are increasingly turning to outsourcing as a means of gaining an advantage. With the many competing Contract Development and Manufacturing Organizations (CDMOs) and Contract Research...
Employee Spotlight: Maria Trance
Employee Spotlight Our employee spotlight series celebrates the accomplishments and expertise of our valued scientists and staff. Maria Trance Maria Trance Senior Operations Manager Q&A with Maria What is your favorite part of your role here as a Senior Operations...
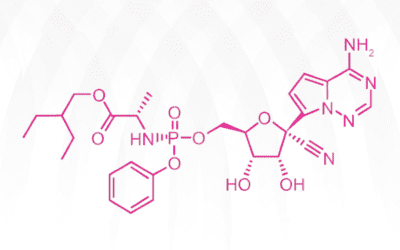
How Does Remdesivir Work?
Gileadʹs antiviral drug remdesivir has been catapulted to the limelight as the only FDA-approved treatment of coronavirus Covid-19 for emergency use thus far. On April 29, 2020, preliminary clinical trials indicated that the drug has shown some efficacy. While...
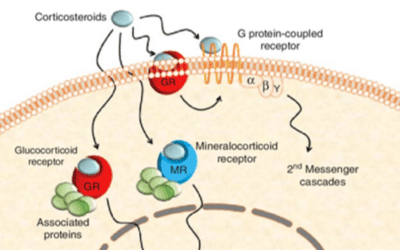
The Story of Dexamethasone
On June 16th, 2020, a team at Oxford University announced that their RECOVERY trials had revealed that a daily treatment of 6 mg of dexamethasone (Decadron, 1) lowered the fatality rate of ventilated COVID-19 patients by up to one third! This was a randomized,...
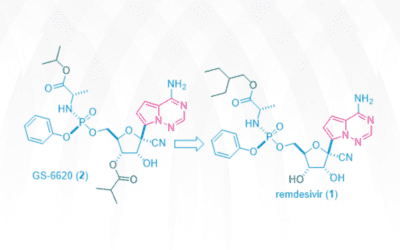
Genesis of Remdesivir
Gileadʹs antiviral drug remdesivir (Veklury) was approved by the FDA on May 1, 2020 for emergency use in coronavirus disease-2019 (COVID-19) patients after a one-day review. It took the Japanese government much longer to give the nod, seven days, before granting its...