Gileadʹs antiviral drug remdesivir (Veklury, 1) was approved by the FDA on May 1st for emergency use against coronavirus disease-2019 (COVID-19) after just a one-day review. Now the onerous task falls upon Gilead to make enough of the drug to supply doctors and...
Search Results
How Does Remdesivir Work?
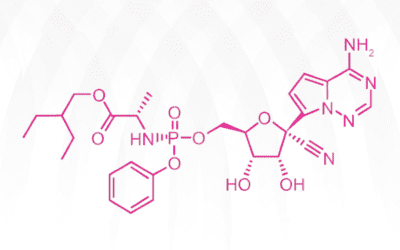
Gileadʹs antiviral drug remdesivir has been catapulted to the limelight as the only FDA-approved treatment of coronavirus Covid-19 for emergency use thus far. On April 29, 2020, preliminary clinical trials indicated that the drug has shown some efficacy. While...
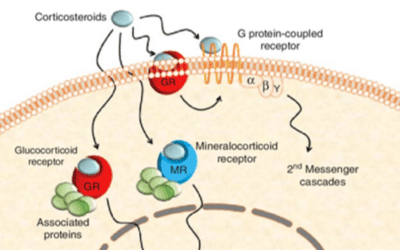
The Story of Dexamethasone
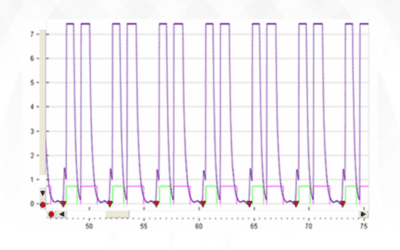
On June 16th, 2020, a team at Oxford University announced that their RECOVERY trials had revealed that a daily treatment of 6 mg of dexamethasone (Decadron, 1) lowered the fatality rate of ventilated COVID-19 patients by up to one third! This was a randomized,...
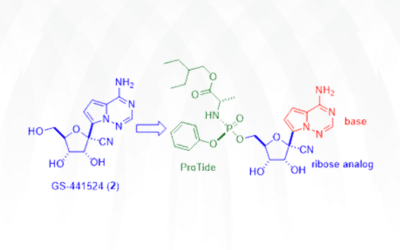
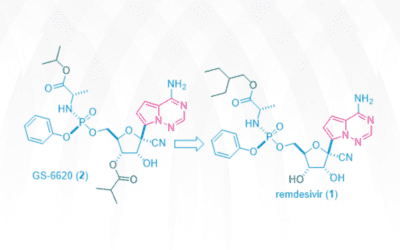
Genesis of Remdesivir
Gileadʹs antiviral drug remdesivir (Veklury) was approved by the FDA on May 1, 2020 for emergency use in coronavirus disease-2019 (COVID-19) patients after a one-day review. It took the Japanese government much longer to give the nod, seven days, before granting its...
Meeting Chiral Separation Efficiency Needs in Pharmaceutical Discovery with Supercritical Fluid Chromatography
Supercritical fluid chromatography (SFC) is now a well-established tool incorporated into the small molecule drug development strategy for organizations of all types and sizes. Its imprint is almost surely on the history of development of any new advanced chiral drug...
Innovations in Lead Generation Through Fragment-Based Screening
When it comes to finding new chemical matter to engage biological targets, fragment-based screening (FBS) offers a number of advantages over traditional high-throughput screening (HTS). The success of this approach has resulted in a growing list of molecules advancing...